Association between HBsAg clearance and serum level of granulocyte-macrophage colony stimulating factor in inactive HBsAg carriers receiving pegylated interferon alpha-2a
-
摘要: 目的探讨聚乙二醇干扰素(PEG-IFN)α-2a治疗非活动性HBs Ag携带者(IHC)发生HBs Ag清除与血清粒-巨噬细胞集落刺激因子(GM-CSF)水平的关系。方法收集2013年1月-2016年1月就诊于首都医科大学附属北京佑安医院门诊的IHC患者20例,经PEG-IFNα-2a治疗24周后13例获得HBs Ag清除(R组),7例未获得HBs Ag清除(NR组)。用Luminex技术检测患者基线、治疗12周和治疗24周血清GM-CSF水平。同时检测11例健康人(HC组)血清GM-CSF水平。计量资料2组间比较采用t检验,多组间比较采用方差分析,进一步两两比较采用Bonferroni检验。计数资料组间比较采用χ2检验。结果 R组、NR组及HC组基线血清GM-CSF水平分别为(42.63±11.24)pg/ml、(46.77±10.52)pg/ml(11.97±3.85)pg/ml,3组之间比较差异具有统计学意义(F=4.482,P=0.02),R组与NR组GM-CSF水平均明显高于HC组(P值均<0.05)。PEG-IFNα-2a治疗后12周和24周,R组血清GM-CSF水平...
-
关键词:
- 肝炎病毒,乙型 /
- 肝炎表面抗原,乙型 /
- 聚乙烯二醇类 /
- 干扰素类 /
- 粒细胞巨噬细胞集落刺激因子
Abstract: Objective To investigate the association between HBs Ag clearance and serum level of granulocyte-macrophage colony stimulating factor ( GM-CSF) in inactive HBs Ag carriers receiving pegylated interferon ( PEG-IFN) alpha-2 a. Methods A total of 20 inactive HBs Ag carriers who visited Beijing You An Hospital, Capital Medical University, from January 2013 to January 2016 were enrolled, and after 24 weeks of PEG-IFN alpha-2 a treatment, 13 achieved HBs Ag clearance ( R group) and 7 did not achieve HBs Ag clearance ( NR group) . The Luminex technique was used to measure the serum level of GM-CSF at baseline and at weeks 12 and 24 of treatment. The serum level of GM-CSF was also measured for 11 healthy controls ( HC group) . The t-test or an analysis of variance was used for comparison of continuous data between groups, and the Bonferroni test was used for further comparison between two groups. The chi-square test was used for comparison of categorical data between groups. Results The R and NR groups had a significantly higher serum GM-CSF level than the HC group ( 42. 63 ±11. 24 pg/ml and 46. 77 ± 10. 52 pg/ml vs 11. 97 ± 3. 85 pg/ml) , and there was a significant difference between the three groups ( F = 4. 482, P = 0. 02) . At weeks 12 and 24 of PEG-IFNα-2 a treatment, the R group had a significantly higher serum GM-CSF level than the NR group ( t = 22. 422 and 17. 782, both P < 0. 05) . In the R group, the serum GM-CSF level was 42. 63 ± 11. 25 pg/ml at baseline, significantly increased to 83. 31 ± 14. 20 pg/ml at week 12 of treatment, and then significantly decreased to 32. 34 ± 8. 06 pg/ml at week 24 of treatment ( F =7. 655, P =0. 002) . In the NR group, the serum GM-CSF level was 46. 77 ± 10. 52 pg/ml at baseline and significantly decreased to25. 90 ± 7. 06 pg/ml at week 12 of treatment and 9. 43 ± 2. 45 pg/ml at week 24 of treatment ( F = 5. 264, P = 0. 016) . Conclusion In inactive HBs Ag carriers receiving PEG-IFN alpha-2 a treatment, the increase in serum GM-CSF level may indicate HBs Ag clearance. -
1995年慢加急性肝衰竭(ACLF)的概念首次被Ohnishi和Muto教授[1]提出后,10多年间,各大学会对ACLF的认识始终停留在专家个人意见或小范围的共识层面。直到2013年,欧洲肝衰竭联盟通过一项前瞻性多中心队列研究,明确提出了ACLF诊断的欧洲标准[2],自此学界对于ACLF的认识有了迅速的拓展。虽然目前在临床实践中,仍有医生质疑这一群体的存在,但亚太肝病学会(APASL)已先后发布3版ACLF临床管理共识[3-5],美国胃肠病学会正进行ACLF指南的撰写,欧洲肝病学会(EASL)2018版的肝硬化失代偿指南也有针对ACLF人群临床管理的推荐意见[6],我国肝衰竭指南也明确划分出了ACLF这一亚型[7]。目前在肝硬化及肝衰竭领域,较为公认的是,ACLF是明显区别于急性肝硬化失代偿和急性肝衰竭的一类临床综合征,其主要特征是慢性肝病基础上遭受一系列诱发炎症的急性事件(如细菌感染、酒精性肝炎或HBV再激活等),形成强烈的系统性炎症反应和多器官功能障碍/衰竭,短期死亡率高。然而由于不同地区病因和诱因的巨大差异,ACLF的临床表现和病程转归存在多种形式,在不同地域的学会中仍存在认识分歧,具体的诊断标准不同,适用人群也不同,落实到临床实践中,甚至同一地区不同医生之间对于“ACLF”的管理路径也不一致。如何应用目前已有的国际标准优化ACLF的临床管理,逐渐实现ACLF管理理念的同质化和标准化是未来的重要方向,更需要坚实的数据来支撑同质化管理路径的建立。
1. ACLF各大国际诊断标准的发展历程
1.1 APASL-ACLF研究联盟(APASL ACLF Research Consortium,AARC)
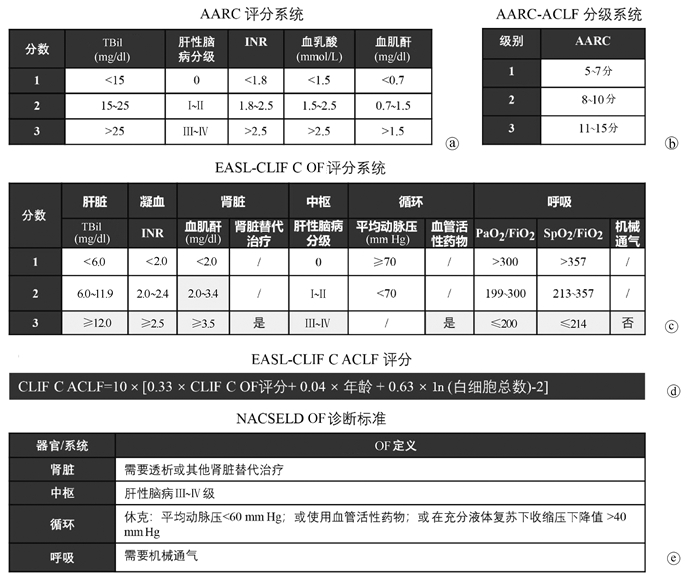
亚太地区对于ACLF的认识最早由Ohnishi和Muto教授[1]于1995年提出,描述了肝脏同时遭受慢性损伤和急性打击的情况。这一人群短期死亡率和急性肝衰竭类似,但由于缺乏归纳总结,当时对于肝衰竭临床上命名和诊治较为混乱。2004年,在Sarin教授[3]的领导下,APASL形成了专门的工作小组对ACLF进行系统研究,通过文献荟萃、专家讨论及回顾性研究(涉及200余例患者)等方式,总结形成了第一版ACLF专家共识(2009版)。2012年进一步建立了APASL-ACLF研究联盟,简称“AARC”,开始了回顾-前瞻性队列的构建,分别于2014年(涉及1402例患者)[4]及2019年(涉及3300例患者)[5]更新共识。最新版本的APASL-ACLF诊断标准及适用人群见表 1。亚太共识对于ACLF的诊断理念是将肝衰竭作为ACLF的始发和核心事件,可导致肝外器官衰竭(organ failure,OF),加速疾病进展。ACLF的主要诱因为肝脏损伤,如HBV再激活、酒精性肝炎等,感染或脓毒症是ACLF的并发症而非诱因。强调早期“黄金窗口期”识别的重要性和ACLF存在可逆转性[8]。2019版共识推出了AARC的评分系统用于APASL-ACLF患者的预后评估(图 1a)。该评分系统涉及的参数有TBil、INR、血清肌酐、血清乳酸和肝性脑病分级,每项参数根据数值大小,取1~3分,总分范围为5~15分,分数越高,预后越差,其预测28 d死亡率的C指数为0.77~0.79[9]。根据总分范围,AARC-ACLF可分为3级,AARC-ACLF-1级: 5~7分; 2级:8~10分; 3级:11~15分(图 1b)。基线AARC-ACLF-1、2、3级的28 d非移植存活率分别为85.9%、45.5%和12.7%(表 1)。
表 1 3大国际ACLF诊断标准特点对比项目 APASL-AARC EASL-CLIF C AASLD-NACSELD 适用人群 慢性肝病或代偿期肝硬化;因肝脏直接的急性损伤,首次出现肝功能急剧恶化 代偿期或失代偿期肝硬化患者;因以下一种或多种急性失代偿事件而住院:腹水,肝性脑病,消化道大出血或急性细菌感染 代偿期或失代偿期患者;因感染入院或住院期间发生感染 排除人群 既往有失代偿史;初次失代偿但住院原因为肝硬化急性失代偿;合并细菌感染;合并肝癌患者 孕妇;因预约的操作或治疗入院;进展期肝癌;严重肝外疾病;HIV感染;正接受非重症酒精性肝炎相关的免疫抑制治疗 门诊患者;既往实体器官移植术后;恶性肿瘤转移 诱因 仅考虑肝脏诱因,如HBV再激活、酒精性肝炎、药物性肝损伤、自身免疫性肝炎发作等 同时考虑肝脏诱因和肝外诱因 仅考虑感染(属于肝外诱因) 诊断标准 慢性肝病或肝硬化代偿期,在急性肝脏打击后,出现黄疸[TBil<5 mg/dl (85 μmol/L)]和凝血功能障碍(INR≥1.5或凝血酶原活动度<40%),发病4周内并发临床显性腹水和/或肝性脑病 根据EASL-CLIF C OF定义(详见图 1c),出现2个或以上OFs,或单个肾衰竭,或单个脑衰竭合并肌酐1.5~1.9 mg/dl,或单个肝/凝血/循环或呼吸衰竭合并肌酐1.5~1.9 mg/dl和/或肝性脑病Ⅰ~Ⅱ级 根据NACSELD OF定义(详见图 1e),出现2个或以上OFs 分层及预后
(28 d死亡率)基于AARC评分(参见图 1)
Grade-1:14.1%
Grade-2:55.5%
Grade-3:87.3%基于OF数
Grade-1:20%
Grade-2:30%
Grade-3:80%基于OF数
2个OFs:49%
3个OFs:64%
4个OFs:77%1.2 EASL慢性肝衰竭联盟(EASL-Chronic Liver Failure Consortium, EASL-CLIF C)
欧洲地区专家对于ACLF的提出稍晚,于2002年由Williams和Jalan教授[10]提出,研究过程中逐渐发现了在肝硬化急性失代偿人群中,部分患者发生以多个OFs为特征的综合征,短期死亡率(入院后28 d)高。2006年波士顿美肝会期间,与北美学者讨论后,决定分头建立肝衰竭联盟。2009年在EASL资助下建成了目前的欧洲慢性肝衰竭联盟,2011年2月开始了欧洲地区的多中心前瞻性研究,并迅速在9个月内完成了来自12个欧洲国家29家肝病中心1343例肝硬化急性失代偿患者的入组,于2013年发表了ACLF领域里程碑式的“CANONIC”研究[2]。该研究采用了序贯器官衰竭评分(sequential organ failure assessment,SOFA)系统,并对部分参数进行修改,形成了适应肝硬化的CLIF-SOFA评分和CLIF C OF评分(图 1c),并预设15%作为28 d死亡率的最小界限值。在这一研究设计下,建立了EASL-CLIF C ACLF诊断标准(表 1)。这一标准对于ACLF的概念强调:(1)一个明确的慢性肝病,即肝硬化形成;(2)两类诱因共存,即肝脏诱因和肝外诱因(如细菌感染、消化道出血等);(3)多个OFs;(4)短期高死亡率。根据OF数目,ACLF可分为3级,ACLF-1、2、3级的28 d死亡率分别为22%、32%和73%。基于“CANONIC”队列,研究者进一步构建了ACLF预后评分,即CLIF-C ACLF评分(图 1d)。该模型预测肝硬化ACLF的28 d和90 d死亡率C指数分别为0.76和0.73,优于CTP、MELD和MELD-NA评分[11]。
1.3 美国肝病学会-北美终末期肝病联盟(American Association for the Study of Liver Diseases-North American Consortium for the Study of End-Stage Liver Disease, AASLD-NACSELD)
北美地区对于ACLF的研究主要由NACSELD组织开展,核心成员包括Patrick Kamath、Florence Wong、Jasmohan Bajaj教授等。该团队于2010年底开始在美国和加拿大18家肝病中心招募病例。起初研究对象限定于肝硬化合并细菌或真菌感染的住院患者,研究目的也主要针对感染进行。截至2014年入组507例患者,构建了NACSELD-ACLF标准(表 1)[12]。这一标准较CLIF C诊断标准减少需要评定的OF数目,简化了评定方式,仅针对肝脏、肾脏、循环系统和中枢系统,将满足2个或以上OFs的患者定义为ACLF。从诱因的角度讲,北美标准的适用人群局限于肝外诱因,细菌感染所诱发的多个OFs,这正好与APASL-AARC观念对立。也解释了为什么关注肝脏诱因的APASL-ACLF标准的核心参数为胆红素和凝血酶原时间,而这两项指标没有被包含在北美的标准中,取而代之的是肝外OF评估(图 1e)。2013年后,北美的学者将NACSELD研究队列进一步扩展到了感染以外因素导致的肝硬化住院患者,截至2018年共入组2675例患者,其中1596例非感染相关,1079例为感染相关。根据NACSELD-ACLF器官衰竭评定体系,合并感染的患者,NACSELD-ACLF的30 d存活率为52%,发生1、2、3、4个OF患者的30 d存活率分别为80%、62%、42%和0;未发生细菌感染的患者,NACSELD-ACLF的30 d存活率为76%,发生1、2、3、4个OF患者的30 d存活率分别为90%、84%、65%和24%[13]。通过多因素分析,研究者在全人群中筛选获得了6个与30 d存活率独立相关的风险因素,包括NACSELD-ACLF、感染、年龄、血清白蛋白、血白细胞计数和MELD评分,由此产生的模型预测30 d存活率的C指数为0.80,但具体的模型计算公式笔者未能查阅到。
1.4 世界消化病组织(World Gastroenterology Organization, WGO)
为了尽可能地统一ACLF的诊断分歧,WGO在2014年组织了东西方ACLF领域的专家代表形成了一份共识文件[14]。WGO将ACLF定义为“慢性肝病、代偿期肝硬化或失代偿期肝硬化基础上因急性肝功能失代偿所导致的肝衰竭(表现为黄疸和凝血酶原时间延长)以及1个或多个肝外OF的临床综合征,3个月死亡率升高”。这一共识将多方的ACLF概念进行整合,形成了一个运作定义,以聚焦一个人群,收集前瞻性数据优化ACLF这一临床综合征的定义,以求在数据充分时制订全球统一的ACLF诊断标准。WGO共识将ACLF分为3个类型:A型-慢性肝病型;B型-代偿期肝硬化型;C型-失代偿期肝硬化型。遗憾的是,在WGO的框架下,ACLF的理念差异没有得到根本的解决,停留在表面的“共识”上。
1.5 其他基于国家指南/共识/队列的ACLF指南
除了APASL、EASL、AASLD 3大学会构建的ACLF诊断标准外,尚有多个利用国家队列构建的ACLF标准。
中国:我国最早于2006年制定了肝衰竭诊疗指南[15],当时就提出了ACLF的概念“在慢性肝病基础上,短期内发生急性肝功能失代偿的主要临床表现”,在2012版指南[16]中进一步给出了定义:(1)极度乏力,有明显的消化道症状;(2)黄疸迅速加深,血清TBil>10倍正常值上限或每日上升≥17.1 μmol/L;(3)出血倾向,凝血酶原活动度≤40%(或INR≥1.5),并排除其他原因者;(4)失代偿性腹水;(5)伴或不伴有肝性脑病。在最新的2018版指南中[7],参照了WGO共识,进一步将ACLF分为A/B/C三型。除此以外,针对我国乙型肝炎流行率较高的特点,研究者在国家科技部重大专项支撑下,协同国内各大省市13家三甲医院1202例重型乙型肝炎及乙型肝炎肝硬化失代偿病例,构建了乙型肝炎相关ACLF的诊断标准(COSSH标准)[17]。这一标准在CLIF标准的基础之上新增了“肝衰竭(TBil≥12 mg/dl)合并凝血障碍(INR 1.5~2.4)”作为ACLF-1级的补充诊断标准,并在非肝硬化慢性乙型肝炎相关ACLF人群中进行了验证。相比CLIF标准,该标准起到了提早预警的作用。
日本:虽然ACLF的概念最早由日本教授提出,但对于ACLF的首个系统研究开始于2015年—2016年间,由日本厚生劳动省(负责医疗卫生和社会保障的主要部门)的难治性肝胆疾病学组牵头完成。这项研究回顾性分析了2011年—2014年间日本8个中心,112例慢性肝病急性失代偿的住院患者,其中绝大部分患者满足APASL-ACLF标准(109例),但对于预后评估,EASL-CLIF C ACLF的分级系统准确性更高[18]。结合日本队列的特征和国情,提出了ACLF的日本标准,即“肝硬化CTP 5~9分的患者在28 d内因各种诱因导致肝功能严重恶化,表现为血清TBil≥5 mg/dl且凝血酶原活动度≤40%和/或INR≥1.5”。这一标准,目前尚未得到大样本队列的前瞻性验证。
2. ACLF的全球负担和差异
由于各大国际肝病学会对于ACLF诊断标准的适用人群和OF定义不同,全球的流行病学数据匮乏。最近,Mezzano等[19]通过荟萃分析全球30项运用EASL-CLIF C ACLF诊断标准的队列研究,在184 041例肝硬化失代偿期患者中,ACLF的估算患病率达35%,90 d估算死亡率为58%。这项研究首次通过EASL-CLIF C ACLF诊断标准展示了目前全球ACLF的疾病卫生负担,也强调了各地区之间同一ACLF诊断标准下,病因、诱因、OF特征的差异。在此基础上,进一步整理了各大标准ACLF患者的28 d或90 d死亡率情况(表 2)[20-32]。整体上,各种诊断标准的ACLF死亡率均较高,28 d死亡率最低为25.5%,最高65%,90 d死亡率最低35.7%,最高94.3%。但是通过对比可以发现,同一个诊断标准在不同国家之间有较为显著的差异,而在同一个队列中,不同诊断标准也存在明显的差异。这些数据进一步强调了在缺乏全球统一ACLF诊断标准的前提下,ACLF的临床管理需要针对不同的人群,结合相对应的循证医学证据,制订适合的管理路径。
表 2 3大国际ACLF诊断标准确诊的ACLF预后对比第一作者及文献 APASL-AARC EASL-CLIF C AASLD-NACSELD 病例来源 28 d
死亡率90 d
死亡率28 d
死亡率90 d
死亡率28 d
死亡率90 d
死亡率Choudhury*[9] 40.5% 49.2% / / / / AARC队列 Moreau*[2] / / 32.8% 51.2% / / CANONIC队列 O’Leary*[13] / / / / 30 d-41.0% / NACSELD队列 Kulkarni*[20] 43.8% / / / / / 印度-ICU Dhiman*[21] 37.0% / 47.4% / / / 印度 Mahmud#[22] 41.9% 56.1% 37.6% 50.4% / / 美国退伍军人管理局数据 Selva Rajoo*[23] / 35.7% / 53.3% / / 新加坡 Amarapurkar*[24] / 43.1% / 62.0% / / 印度 Shi#[25] / / 49.4% 63.0% / / 中国 Lee#[26] / / 50.4% 66.1% / / 韩国 Silva*[27] / / 30 d-65.0% 69.9% / / 巴西 Picon*[28] / / 61.1% 83.3% / / 巴西 Piano#[29] / / / 45.0% / / 意大利 Meersseman#[30] / / / 40.0% / / 比利时-ICU Hernaez#[31] / / 25.5% 40.2% 33.0% 47.5% 美国退伍军人管理局数据 Cao*[32] / / 41.6% 62.9% 62.9% 94.3% 中国 注:考虑到单病因或单诱因相关的ACLF代表性较弱,因此未纳入总结。*前瞻性设计;#回顾性设计。 3. 目前已发表的ACLF临床管理路径
3.1 APASL-AARC ACLF临床管理路径
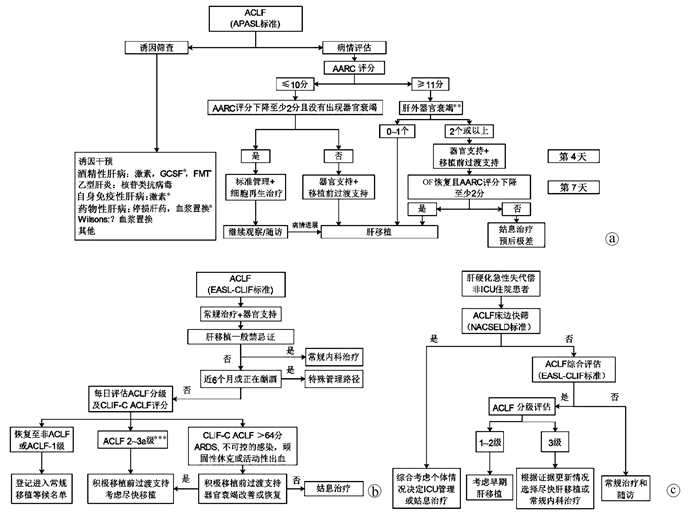
2019版APASL-ACLF共识给出了运用AARC评分优化ACLF管理的临床路径[5](图 2a)。在常规病因和诱因干预治疗基础上,路径中将AARC评分10分作为截断值,针对(1)基线AARC≤10分的患者,如果第4天评估下降≥2分且没有出现OF,则进行常规治疗+肝再生治疗、密切随访等管理路径;若第4天评估AARC较基线下降不足2分或出现OF,启动器官支持及人工肝等肝移植前过渡治疗,有条件者择期行肝移植术。针对(2)基线AARC≥11分的患者,如果基线只有1个或没有肝外OF,进入肝移植路径;若基线存在2个或以上肝外OFs,首先进行器官支持和肝移植前过渡治疗,第7天评估若OF逆转且AARC评分减少≥2分,进入肝移植路径;若第7天评估未满足上述条件,预后极差,推荐姑息治疗。
 图 2 ACLF临床管理路径注:a,APASL-2019版ACLF共识对于ACLF临床路径的推荐[5];b,欧洲学者基于CLIF C系列研究结果对基于EASL-CLIF C ACLF的临床管理路径[33, 39];c,肝硬化急性失代偿非ICU住院患者联合运用NACSELD和EASL-CLIF C ACLF的优化管理,数据来源于我国前瞻性队列,乙型肝炎病例为主,对于移植、ICU管理和姑息治疗方面的推荐尚需更多研究数据[32]。GCSF, 粒细胞集落刺激因子;FMT, 粪菌移植;ARDS, 急性呼吸窘迫综合征。* 需要更多证据;* * 肝外OF评估依据SOFA评分;* * * ACLF-3a级为3个OFs的ACLF,ACLF-3b为4~6个OFs的ACLF。
图 2 ACLF临床管理路径注:a,APASL-2019版ACLF共识对于ACLF临床路径的推荐[5];b,欧洲学者基于CLIF C系列研究结果对基于EASL-CLIF C ACLF的临床管理路径[33, 39];c,肝硬化急性失代偿非ICU住院患者联合运用NACSELD和EASL-CLIF C ACLF的优化管理,数据来源于我国前瞻性队列,乙型肝炎病例为主,对于移植、ICU管理和姑息治疗方面的推荐尚需更多研究数据[32]。GCSF, 粒细胞集落刺激因子;FMT, 粪菌移植;ARDS, 急性呼吸窘迫综合征。* 需要更多证据;* * 肝外OF评估依据SOFA评分;* * * ACLF-3a级为3个OFs的ACLF,ACLF-3b为4~6个OFs的ACLF。3.2 EASL-CLIF C ACLF临床管理路径
在CANONIC系列研究中,研究者发现入院后48 h内和1周内发生EASL- CLIF C ACLF分级改变比例分别为40%和80%,根据基线ACLF的分级进行管理无法适应一个快速进展的疾病,为此提出了运用确诊后第3~7天ACLF分级进行常规内科治疗、肝移植、ICU和姑息治疗的分层管理[33]。肝移植是目前ACLF唯一能够改变预后的治疗手段,一直以来ACLF-3级被认为病情太重,不适合移植,在当时CANONIC队列中同时有4~6个OFs或CLIF-C ACLF患者180 d的存活率为0,因此被推荐为姑息治疗。但在2017年欧洲的一项小样本的回顾性研究中发现,ACLF-3级接受移植的患者1年存活率可达83.6%[34],这部分患者肝移植等候期间的死亡率显著高于目前分配优先级最高的1a类患者[35]。这一结果提示,基于MELD-NA评分的器官共享联合网络的肝移植分配体系对ACLF患者不利,因为MELD-NA评分会低估ACLF预后[36]。最新数据表明,即便是4~6个OFs的ACLF-3级患者移植后1年存活率仍有81%~84%[37],在等候移植期间,如果ACLF-3级OF好转,尤其是循环、中枢和呼吸系统衰竭的扭转有利于移植后生存[38],因此在最新的EASL-CLIF C ACLF管理路径中,已经推荐ACLF-3a级(3 OFs)进入优先肝移植队列,推荐ACLF-3b级(4~6 OFs)或CLIF C ACLF评分>64分或存在ARDS等患者在积极移植前过渡治疗好转后尽快进行肝移植[39](图 2b)。
3.3 肝硬化急性失代偿非ICU住院患者的NACSELD & CLIF C ACLF联合管理路径
虽然,早在2013年—2014年欧美就已经在肝硬化住院患者中建立了ACLF诊断标准,但由于理念分歧,这两个标准始终未能落实在临床实践中优化患者管理。为此,笔者团队[32]通过前瞻性队列,直接对比了北美NACSELD和欧洲EASL-CLIF C ACLF标准在肝硬化非ICU住院患者中预后预测特点,发现NACSELD标准准确性高,其预测28 d死亡的特异度高达99.7%,但敏感性低。相比之下EASL-CLIF标准的优势在于早期预警,其预测28 d死亡的敏感度为92.5%。序贯运用NACSELD与EASL-CLIF标准,可在现有标准基础上最大化利用两种标准的优点,进一步优化肝移植、ICU监护、姑息治疗等临床决策,实现更为精准的肝病终末期管理(图 2c)。
4. 总结
ACLF目前仍然是一个不能被确切定义的疾病,但随着研究不断的深入,层层面纱被不断揭开。纵然东西方学界对于ACLF的诊断和管理理念存在差异,但都从不同的角度在拓展这一肝病中的核心重症人群。ACLF已然是一个全球的卫生负担,不断挑战着肝脏、消化、感染、重症、移植等学科的医护团队。相比欧美ACLF研究的进展,目前我国在ACLF管理各个关键环节,如肝移植、ICU和姑息治疗方面数据仍较为有限,由于人群特征以及国情和医疗条件与西方存在差异,进一步加强相关学科、地区之间的合作,总结数据形成中国ACLF管理的循证医学证据链,是未来优化临床实践的必经之路。
-
[1] Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association.The guideline of prevention and treatment for chronic hepatitis B:a 2015 update[J].J Clin Hepatol, 2015, 31 (12) :1941-1960. (in Chinese) 中华医学会肝病学分会, 中华医学会感染病学分会.慢性乙型肝炎防治指南 (2015年更新版) [J].临床肝胆病杂志, 2015, 31 (12) :1941-1960. [2]TERRAULT NA, BZOWEI NH, CHANG KM, et al.AASLD guidelines for treatment of chronic hepatitis B[J].Hepatology, 2016, 63 (1) :261-283. [3] SARIN SK, KUMAR M, LAU GK, et al.Asian-Pacific clinical practice guidelines on the management of hepatitis B:a 2015 update[J].Hepatol Int, 2016, 10 (1) :1-98. [4]CHU CM, LIAW YF.Spontaneous relapse of hepatitis in inactive HBs Ag carriers[J].Hepatol Int, 2007, 1 (2) :311-315. [5]CHEN JD, YANG HI, IIOEIE UH, et al.Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liverrelated death[J].Gastroenterology, 2010, 138 (5) :1747-1754. [6]CHUNG SJ, KIM JK, PARK MC, et al.Reactivation of hepatitis B viral infection in inactive HBs Ag carriers following anti-tumor necrosis factor-alpha therapy[J].J Rheumatol, 2009, 36 (11) :2416-2420. [7]HWANG JP, LOK AS.Management of patients with hepatitis B who require immunosuppressive therapy[J].Nat Rev Gastroenterol Hepatol, 2014, 11 (4) :209-219. [8]ZHOU K, TERRAULT N.Management of hepatitis B in special populations[J].Best Pract Res Clin Gastroenterol, 2017, 31 (3) :311-320. [9]CAO Z, LIU Y, MA L, et al.A potent hepatitis B surface antigen response in subjects with inactive hepatitis B surface antigen carrier treated with pegylated-interferon alpha[J].Hepatology, 2017, 66 (4) :1058-1066. [10]HABERSETZER F, MOENNE-LOCCOZ R, MEYER N, et al.Loss of hepatitis B surface antigen in a real-life clinical cohort of patients with chronic hepatitis B virus infection[J].Liver Int, 2015, 35 (1) :130-139. [11]OLIVERI F, SURACE L, CAVALLONE D, et al.Long-term outcome of inactive and active, low viraemic HBe Ag negative-hepatitis B virus infection:benign course towards HBs Ag clearance[J].Liver International, 2017, 37 (11) :1622-1631. [12]GUARDIOLA ARVALO A, GMEZ RODRGUEZ R, ROMERO GUTIRREZ M, et al.Characteristics and course of chronic hepatitis B e antigen-negative infection[J].Gastroenterol Hepatol, 2017, 40 (2) :59-69. [13]ZACHARAKIS GH, KOSKINAS J, KOTSIOU S, et al.Natural history of chronic HBV infection:a cohort study with up to12 years follow-up in North Greece (part of the Interreg III/EC-project) [J].J Med Virol, 2005, 77 (2) :173-179. [14]CHU CM, LIAW YF.Incidence and risk factors of progression to cirrhosis in inactive carriers of hepatitis B virus[J].Am J Gastroenterol, 2009, 104 (7) :1693-1699. [15]WANG X, DONG A, XIAO J, et al.Overcoming HBV immune tolerance to eliminate HBs Ag-positive hepatocytes via pre-administration of GM-CSF as a novel adjuvant for a hepatitis B vaccine in HBV transgenic mice[J].Cell Mol Immunol, 2016, 13 (6) :850-861. [16]ROOZBEH J, BAGHERI-LANKARANI K, MOHAGHEGH P, et al.A randomized pilot trial on the effect of granulocyte-colony stimulating factor on antibody response in hemodialysis patients who had not responded to routine hepatitis B virus vaccine[J].J Nephropathol, 2015, 4 (1) :13-17. [17]QING Y, CHEN M, ZHAO J, et al.Construction of an HBV DNA vaccine by fusion of the GM-CSF gene to the HBV-S gene and examination of its immune effects in normal and HBV-transgenic mice[J].Vaccine, 2010, 28 (26) :4301-4307. [18]YU GD.New regimens for antiviral/immunoregulatory therapy in treatment-naive patients with HBe Ag-positive chronic hepatitis B[D].Hangzhou:Zhejiang Univ, 2016. (in Chinese) 俞国栋.HBe Ag阳性慢性乙型肝炎初治患者抗病毒/免疫调节治疗新方案的研究[D].杭州:浙江大学, 2016. [19]CAI H.New regimens for antiviral therapy/immunotherapy reduce ccc DNA level in hepatocytes in treatment-naive patients with HBe Ag-positive chronic hepatitis B[D].Hangzhou:Zhejiang Univ, 2016. (in Chinese) 蔡欢.抗病毒/免疫治疗新方案降低HBe Ag阳性慢乙肝初治患者肝细胞ccc DNA水平研究[D].杭州:浙江大学, 2016. [20] CHI CP.WANG YM, WANG YF, et al.A preliminary study on the clinical effect of IFNα-2b combined with rh GM-CSF in treatment of chronic hepatitis B[C].The 14th National Academic Conference on Interferons and Cytokines, 2004. (in Chinese) 迟春萍, 王玉梅, 王艳芬, 等.IFNα-2b联合rh GM-CSF治疗慢性乙肝的初步临床研究[C].第14次全国干扰素及细胞因子学术会议, 2004. [21]ZHAO W, ZHOU X, ZHAO G, et al.Enrichment of Ly6Chi monocytes by multiple GM-CSF injections with HBV vaccine contributes to viral clearance in a HBV mouse model[J].Hum Vaccin Immunother, 2017, 13 (12) :2872-2882. 期刊类型引用(3)
1. 刘磊,梁静,徐佰国,王菲,连佳,杨言开. 再代偿乙型肝炎肝硬化患者发生慢加急性肝衰竭的临床特点及预后分析. 临床肝胆病杂志. 2023(01): 70-76 .  本站查看
本站查看2. 刘斐,杨倩倩,武瑞,刘春涛,傅晓晴,刘寿荣. 基于炎症标志物的预测模型在肝衰竭患者短期预后评估中的应用价值研究. 中国实用内科杂志. 2023(07): 583-588 .  百度学术
百度学术3. 杨阳,陈丹. 血清免疫球蛋白检验对肝衰竭的诊断价值研究. 中国实用医药. 2023(17): 73-76 .  百度学术
百度学术其他类型引用(3)
-

 本文二维码
本文二维码
计量
- 文章访问数: 2232
- HTML全文浏览量: 12
- PDF下载量: 406
- 被引次数: 6



 PDF下载 ( 1650 KB)
PDF下载 ( 1650 KB)

 下载:
下载:

 百度学术
百度学术 下载:
下载: