Value of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in prognostic evaluation of patients with long-term survival after radiofrequency ablation for hepatocellular carcinoma
-
摘要: 目的探讨血小板与淋巴细胞比值(PLR)、中性粒细胞与淋巴细胞比值(NLR)对射频消融术后生存期超过5年的肝细胞癌(HCC)患者预后评估价值。方法选取2006年6月-2012年2月于首都医科大学附属北京佑安医院行经肝动脉化疗栓塞术联合射频消融治疗且生存期超过5年的肝细胞癌患者135例。收集所有患者基线实验室及影像检查资料,根据血常规结果计算NLR与PLR。计数资料组间比较采用χ2检验。采用受试者工作特征曲线(ROC曲线)确定NLR、PLR临界值,Kaplan-Meier法绘制生存曲线,log-rank检验比较生存率,将log-rank检验分析中具有统计学差异的指标纳入Cox多因素分析。结果根据ROC曲线,确定NLR临界值为2. 08,PLR临界值为96. 82。按照治疗前NLR、PLR临界值分为:低NLR组(NLR <2. 08,n=60)与高NLR组(NLR≥2. 08,n=75),低PLR组(PLR <96. 82,n=78)与高PLR组(PLR≥96. 82,n=57),结果显示低NLR组与高NLR组患者AFP、巴塞罗那分期差异均有统计学意义(χ<...
-
关键词:
- 癌,肝细胞 /
- 导管消融术 /
- 血小板与淋巴细胞比值 /
- 中性粒细胞与淋巴细胞比值 /
- 预后 /
- 危险因素
Abstract: Objective To investigate the value of platelet-to-lymphocyte ratio ( PLR) and neutrophil-to-lymphocyte ratio ( NLR) in the prognostic evaluation of hepatocellular carcinoma ( HCC) patients with a survival time of > 5 years after radiofrequency ablation. Methods A total of 135 HCC patients who underwent transcatheter arterial chemoembolization combined with radiofrequency ablation in Beijing YouAn Hospital, Capital Medical University, from June 2006 to February 2012 and had a survival time of > 5 years were enrolled. Baseline laboratory and imaging data were collected, and NLR and PLR were calculated based on routine blood test results. The chi-square test was used for comparison of categorical data between groups. The receiver operating characteristic ( ROC) curve was used to determine the cut-off values of NLR and PLR; the Kaplan-Meier method was used to plot survival curves, and the log-rank test was used to compare survival rates; the indices with statistical differences in the log-rank test were included in the Cox multivariate analysis. Results According to the ROC curve, the cut-off value of NLR was 2. 08 and that of PLR was 96. 82. According to the cut-off values of NLR and PLR before treatment, the patients were divided into low NLR group ( NLR < 2. 08, 60 patients) and high NLR group ( NLR≥2. 08, 75 patients) , as well as low PLR group ( PLR < 96. 82, 78 patients) and high PLR group ( PLR≥96. 82, 57 patients) . There were significant differences between the low NLR group and the high NLR group in alpha-fetoprotein ( AFP) and BCLC stage ( χ2= 15. 125 and 9. 649, both P < 0. 05) , and there were significant differences between the low PLR group and the high PLR group in AFP, cholinesterase ( ChE) , BCLC stage, and tumor size ( χ2= 25. 511, 4. 220, 9. 265, and 16. 403, P < 0. 05) . The low NLR group had a significantly higher survival rate than the high NLR group ( χ2= 31. 302, P < 0. 01) , and the low PLR group had a significantly higher survival rate than the high PLR group ( χ2=92. 905, P < 0. 01) . The Cox multivariate analysis showed that preoperative PLR ( odds ratio [OR] = 9. 634, 95% confidence interval[CI]5. 167-17. 964, P < 0. 001) , ChE ( OR = 0. 404, 95% CI: 0. 236-0. 692, P = 0. 001) , tumor diameter ( OR = 3. 861, 95% CI:1. 760-8. 472, P = 0. 001) , and BCLC stage ( OR = 9. 607, 95% CI: 1. 228-75. 151, P = 0. 031) were independent influencing factors for the survival rate of HCC patients with a long-term survival time ( > 5 years) after radiofrequency ablation. Conclusion PLR is an independent risk factor for survival rate of HCC patients with a long-term survival time after radiofrequency ablation, and the prognosis tend to become worse with the increase in PLR. With reference to tumor conditions, it can be used as an important prognostic indicator for the long-term survival of HCC patients after radiofrequency ablation. -
肝细胞癌(HCC)是我国最常见的恶性肿瘤之一。国家癌症中心最新数据显示,我国每年新发肝癌约37万人,约32.6万人死于肝癌,其中94.7%与慢性HBV感染有关[1-2]。早期发现、早期诊断和早期治疗是改善原发性肝癌预后的关键。然而目前尚缺乏原发性肝癌高敏感度和高特异度的诊断指标。自噬是细胞自我吞噬胞内长寿蛋白或受损细胞器并通过溶酶体途径进行的分解代谢过程,从而维持细胞稳态[3]。近年研究[4-7]显示自噬在HBV感染和HCC的发生发展起重要作用。但目前关于自噬相关蛋白在HBV相关HCC(HBV-HCC)患者血清中的表达及意义鲜有报道。本研究通过分析HBV-HCC、非HBV相关HCC(nonHBV-HCC)、慢性乙型肝炎(CHB)和健康对照者血清自噬相关蛋白7(ATG7)的表达水平,探讨ATG7对HBV-HCC的诊断价值。
1. 资料与方法
1.1 研究对象
选取2018年6月—2020年12月于本院住院的HCC患者89例。HCC的诊断标准符合《原发性肝癌诊疗规范(2017年版)》[8]并经病理确认。排除标准如下:病理类型为肝内胆管癌或HCC-肝内胆管癌混合型者;已接受过肝移植术、局部消融、肝动脉化疗栓塞术、放化疗等抗肿瘤治疗者;继发性HCC患者;同时伴有其他肿瘤患者。CHB诊断符合《慢性乙型肝炎防治指南(2019年版)》[9]。根据血清是否检出HBsAg和/或HBV DNA分为HBV-HCC组及nonHBV-HCC组。选取同期50例CHB患者(CHB组)和20例健康体检者作为对照组(HC组)。
1.2 资料收集
收集研究对象性别和年龄,总蛋白(TP)、白蛋白(Alb)、TBil、DBil、ALT、AST、GGT和ALP等实验室指标。另收集HBV-HCC组患者的BCLC分期和肿瘤直径等病理资料。
1.3 血清ATG7检测
所有研究对象清晨空腹肘静脉采血,采血后1 h内送实验室,3000 r/min离心10 min分离血清,-80 ℃冰箱保存。ATG7检测前4 h室温下复溶。使用上海江莱生物技术有限公司人ATG7酶联免疫吸附测定试剂盒(批号:Aug 2020)进行检测,严格按试剂盒说明书操作,在加入终止液15 min内用PHOMO酶标仪(安图生物,合肥)在450 nm波长测定各孔光密度(OD值),用WPS表格以标准品的浓度为纵坐标,OD值为横坐标绘制标准曲线并取得多项式公式,根据多项式公式计算每个样本的ATG7浓度。
1.4 伦理学审查
本研究方案经由福建医科大学孟超肝胆医院伦理委员会审批,批号:科审2020-029-01。
1.5 统计学方法
采用SPSS 22.0进行统计分析。非正态分布的计量资料以M(P25~P75)表示,多组间比较采用Kruskal-Wallis H检验,2组间比较采用Mann-Whitney U检验;计数资料组间比较采用χ2检验;采用Spearman进行相关性分析。运用GraphPad Prism7.0绘制散点图,采用Medcalc18.11.3绘制受试者工作特征曲线(ROC曲线)并比较不同指标曲线下面积(AUC)。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
HCC、CHB及HC 3组比较,男女比例差异无统计学意义,其余指标差异均有统计学意义(P值均<0.01)(表 1)。89例HCC患者中,67例(75.28%)血清HBsAg和/或HBV DNA阳性,纳入HBV-HCC组;剩余22例(24.72%)纳入nonHBV-HCC组,包括5例酒精性肝硬化,8例非酒精性脂肪肝,1例慢性丙型肝炎,2例肝硬化(病因未明),6例慢性肝炎(病因未明)。HBV-HCC组血清TBil水平及具有肝硬化背景病例数高于nonHBV-HCC组,而nonHBV-HCC组的血清AST水平和最大肿瘤直径高于HBV-HCC组,差异均有统计学意义(P值均<0.05),其余参数差异均无统计学意义(P值均>0.05)(表 2)。
表 1 3组人口学和实验室特征比较参数 HCC组(n=89) CHB组(n=50) HC组(n=20) χ2值 P值 年龄(岁) 56.0(48.0~66.5) 42.0(32.8~52.5) 37.5(30.0~48.8) 38.807 <0.001 男性[例(%)] 77(86.52) 38(76.00) 13(65.00) 5.480 0.076 TBil(μmol/L) 19.30(12.65~26.10) 19.55(12.78~29.28) 12.15(9.48~18.63) 10.478 0.005 DBil(μmol/L) 7.60(4.70~10.85) 4.45(2.20~7.05) 5.30(4.15~6.35) 17.168 <0.001 TP(g/L) 61.0(54.5~67.5) 66.5(60.8~73.3) 72.0(70.0~77.0) 34.168 <0.001 Alb(g/L) 34.0(31.0~38.5) 37.5(34.0~41.0) 44.0(41.0~47.0) 42.516 <0.001 ALT(U/L) 115.0(62.0~218.5) 87.5(26.5~206.8) 19.0(12.3~26.5) 37.212 <0.001 AST(U/L) 149.0(54.0~286.0) 57.0(31.5~110.5) 17.0(14.3~20.0) 54.345 <0.001 GGT(U/L) 52.0(34.0~110.5) 68.5(29.3~112.0) 17.0(14.3~24.5) 31.527 <0.001 ALP(U/L) 81.0(60.5~103.0) 102.0(82.5~117.0) 66.5(52.8~88.3) 21.160 <0.001 AFP(ng/mL) 37.90(5.00~712.32) 7.20(3.05~241.27) 2.85(2.18~4.48) 33.048 <0.001 ATG7(ng/mL) 21.11(17.76~22.73) 19.21(16.65~20.82) 13.82(8.70~17.82) 33.134 <0.001 表 2 HBV-HCC组与nonHBV-HCC组生化和病理特征比较参数 HBV-HCC组(n=67) nonHBV-HCC组(n=22) 统计值 P值 年龄(岁) 55.0(46.0~ 65.0) 63.5(50.8~71.8) U=535.000 0.055 男/女(例) 58/9 19/3 χ2=0.001 0.981 TBil(μmol/L) 20.60(13.30~27.40) 16.00(11.15~21.48) U=524.500 0.043 DBil(μmol/L) 7.70(4.80~10.90) 7.15(3.80~9.65) U=602.500 0.201 TP(g/L) 61.0(50.0~67.0) 58.5(51.3~68.3) U=638.500 0.348 Alb(g/L) 34.0(31.0~38.0) 33.0(30.3~39.0) U=709.500 0.793 ALT(U/L) 95.0(54.0~198.0) 152.0(89.8~268.0) U=557.000 0.087 AST(U/L) 136.0(37.0~240.0) 236.5(93.8~348.8) U=523.000 0.042 GGT(U/L) 51.0(30.0~111.0) 58.0(35.0~113.3) U=658.000 0.452 ALP(U/L) 76.0(60.0~99.0) 92.0(62.8~127.8) U=575.500 0.124 AFP(ng/mL) 58.00(7.10~945.80) 18.28(2.48~405.33) U=536.500 0.057 BCLC(A/B/C/D,例) 51/4/12/0 12/5/5/0 χ2=5.229 0.073 最大肿瘤直径(cm) 3.95(2.50~7.70) 8.00(3.70~11.00) U=520.000 0.047 肝硬化[例(%)] 55(82.09) 9(40.91) χ2=13.904 <0.001 2.2 HBV-HCC血清ATG7表达水平升高
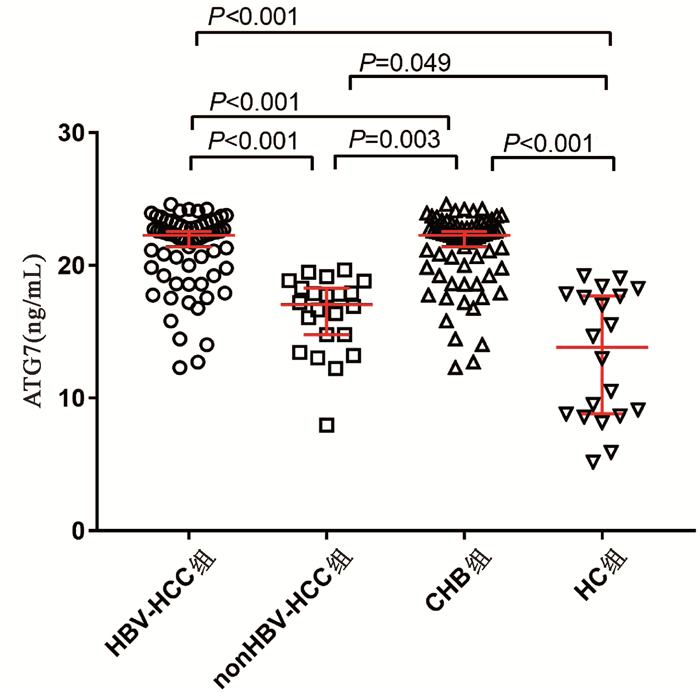
HBV- HCC组、nonHBV-HCC组、CHB组和HC组血清ATG7水平分别为22.88(19.79~23.04)ng/mL、17.06(14.45~19.40)ng/mL、19.21(16.65~20.82)ng/mL和13.82(8.70~17.82)ng/mL,差异有统计学意义(χ2=65.144,P<0.001)。两两比较显示HBV-HCC组血清ATG7不仅高于CHB组(U=758.5,P<0.001)和HC组(U=94.0,P<0.001),也高于nonHBV-HCC组(U=142.0,P<0.001);而nonHBV-HCC组血清ATG7水平不仅低于HBV-HCC组,也低于CHB组(U=311.0,P=0.003),仅稍高于HC组(U=142.0,P=0.049)(图 1)。此外,在67例HBV-HCC患者中,有肝硬化背景和无肝硬化背景患者的血清ATG7水平分别为22.32 (19.79~22.99)ng/mL和20.89(19.40~23.37)ng/mL,差异无统计学意义(P>0.05)。
2.3 血清ATG7表达水平和血清AFP水平的相关性
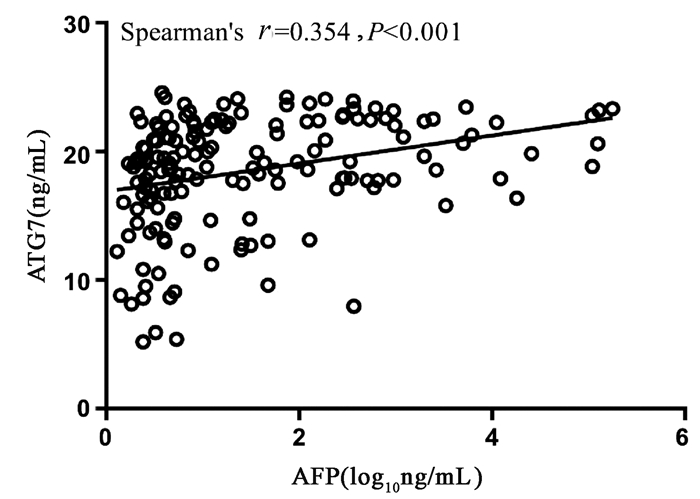
Spearman相关性分析显示,血清ATG7表达水平和血清AFP水平呈正相关,但相关性较弱(r=0.354,95%CI:0.205~0.486,P<0.001)(图 2)。
2.4 ATG7在HBV-HCC中的诊断性能
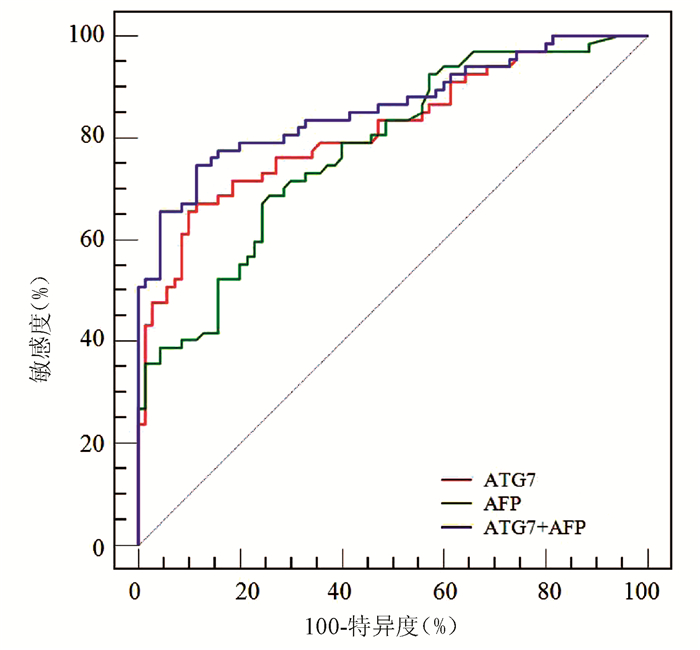
ROC曲线分析显示,ATG7诊断HBV-HCC的AUC为0.818(95% CI:0.743~0.879),稍高于AFP(AUC=0.777,95%CI:0.698~0.843),但二者差异无统计学意义(Z=0.852,P=0.394)(图 3);ATG7诊断HBV-HCC的cut-off值为20.08 ng/mL,敏感度和特异度分别为71.64%和77.14%,均略高于AFP(68.66%和74.29%)。ATG7联合AFP的二元logistic回归预测概率的AUC为0.859 (95%CI:0.790~0.913),显著高于ATG7(Z=2.192,P=0.028)和AFP(Z=2.076,P=0.038)(图 3, 表 3)。
表 3 ATG7、AFP及其联合检测诊断HBV-HCC的性能项目 AUC cut-off值 敏感度(%) 特异度(%) 阳性预测值(%) 阴性预测值(%) AFP 0.777 12.20 ng/mL 68.66 74.29 71.9 71.2 ATG7 0.818 20.08 ng/mL 71.64 77.14 75.0 74.0 ATG7+AFP 0.859 0.56 74.63 88.57 86.2 78.5 3. 讨论
本研究通过ELISA技术检测HCC患者血清ATG7表达水平,结果显示HBV-HCC患者血清ATG7显著升高,血清ATG7是诊断HBV-HCC的良好标志物,ATG7与AFP联合诊断可显著提高HBV-HCC的诊断效率。
ATG7作为一种泛素样修饰物激活酶,通过激活ATG8和ATG12调节自噬体的延伸,是自噬体形成过程的关键蛋白之一[10]。近年研究[11-12]提示ATG7及自噬在HBV-HCC发生发展中发挥重要作用。首先,HBV感染或HBV病毒蛋白的表达可诱导ATG7表达及自噬反应。本研究中CHB组血清ATG7显著高于健康对照组,印证了自噬与HBV感染有关。其次,多项研究[13-14]显示包括ATG7在内的多种自噬相关蛋白在HCC组织表达显著提高。近年的分子细胞学试验进一步揭示多种分子可通过ATG7调节自噬促进HCC发生发展[15-17]。研究[15]显示长链非编码RNA HOX转录反义RNA(HOTAIR)可通过上调ATG7表达激活自噬促进HCC细胞系增殖。癌性锚蛋白重复序列可直接与胞质中的ATG7蛋白相互作用诱导自噬从而促进HCC细胞进展[16]。肿瘤坏死因子α诱导蛋白8(TNFAIP8)则通过与ATG3-ATG7复合物相互作用调节自噬促进HCC细胞增殖[17]。相反,靶向敲减ATG7可抑制自噬,从而促进细胞凋亡、延缓细胞周期及抑制HCC细胞增殖[13]。上述研究结果表明ATG7与HBV-HCC密切相关。本研究数据显示HBV-HCC患者血清ATG7显著高于CHB和健康对照组,进一步证实了自噬与HBV-HCC的相关性,提示血清ATG7是一种潜在HBV-HCC标志物。而且HBV-HCC患者血清ATG7也显著高于nonHBV-HCC,提示不同病因的HCC发病机制不同,其血清标志物也可不同。
虽然AFP已在临床应用数十年,但诊断敏感度不高。最近的一项系统综述[18]显示,AFP诊断HCC的合并敏感度仅为63.9%。与此相符,本研究中AFP诊断HBV-HCC的敏感度、特异度和AUC分别为68.66%、74.29%和0.777。虽然ATG7的敏感度与AFP相仿,但其特异度较高,总体诊断性能较好。此外,ATG7和AFP相关性较弱,提示二者联合检测可有效提高诊断性能。早前有学者[19]提出应用logistic回归分析有助于提高多指标联合诊断效率。本研究结果显示ATG7联合AFP的二元logistic回归预测模型对HBV-HCC的整体诊断性能较好,AUC及诊断敏感度和特异度均显著高于ATG7及AFP。
总之,本研究显示ATG7是HBV-HCC的良好标志物,与AFP有较好的互补性,二者联合检测可显著提高HBV-HCC的诊断效率。然而本研究为单中心横断面研究,纳入研究病例有限,因此其诊断性能仍有待进一步扩大样本的多中心临床验证。
-
[1] PETRUZZIELLO A. Epidemiology of hepatitis B virus (HBV) and hepatitis C virus (HCV) related hepatocellular carcinoma[J]. Open Virol J, 2018, 12:26-32. [2] LI XC, WANG HW, LI CX. Present situation and prospect of comprehensive treatment of hepatocellular carcinoma[J].Chin J Dig Surg, 2018, 17 (5) :433-436. (in Chinese) 李相成, 王宏伟, 李长贤.肝癌综合治疗的现状与展望[J].中华消化外科杂志, 2018, 17 (5) :433-436. [3] ZHAO PC, XU LL, LI YL. Effects and safety of target controlled infusion of propofol combined with dexmetomidine in radiofrequency ablation of primary liver cancer[J]. Chin J Clin Pharmacol Ther, 2018, 23 (6) :682-687. (in Chinese) 赵鹏程, 徐丽丽, 李艳玲.右美托咪定联合靶控输注丙泊酚在肝癌射频消融治疗中的临床效果[J].中国临床药理学与治疗学, 2018, 23 (6) :682-687. [4] NI GY. Sensitivity and specificity of NLR combined with CYFRA21-1 in the early diagnosis of elderly tongue squamous cell carcinoma[J]. Trauma Crit Med, 2018, 6 (2) :93-95. (in Chinese) 倪国宇.中性粒细胞/淋巴细胞比值结合细胞角蛋白19片段对老年舌鳞癌诊断价值研究[J].创伤与急危重病医学, 2018, 6 (2) :93-95. [5] CHEN TM, LIN CC, HUANG PT, et al. Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation[J]. J Gastroenterol Hepatol, 2012, 27 (3) :553-561. [6] PANG Q, ZHOU L, QU K, et al. Validation of inflammationbased prognostic models in patients with hepatitis B-associated hepatocellular carcinoma:A retrospective observational study[J]. Eur J Gastroenterol Hepatol, 2018, 30 (1) :60-70. [7] National Health and Family Planning Commission of the People's Republic of China. Diagnosis, management, and treatment of hepatocellular carcinoma (V2017) [J]. J Clin Hepatol, 2017, 33 (8) :1419-1431. (in Chinese) 中华人民共和国国家卫生和计划生育委员会.原发性肝癌诊疗规范 (2017年版) [J].临床肝胆病杂志, 2017, 33 (8) :1419-1431. [8] DIAKOS CI, CHARLES KA, MCMILLAN DC, et al. Cancerrelated inflammation and treatment effectiveness[J]. Lancet Oncol, 2014, 15 (11) :e493-e503. [9] SHIRAI Y, SHIBA H, SAKAMOTO T, et al. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection[J].Surgery, 2015, 158 (2) :360-365. [10] WEN J, BEDFORD M, BEGUM R, et al. The value of inflammation based prognostic scores in patients undergoing surgical resection for oesophageal and gastric carcinoma[J]. J Surg Oncol, 2018, 117 (8) :1697-1707. [11] GRIVENNIKOV SI, GRETEN FR, KARIN M. Immunity, inflammation, and cancer[J]. Cell, 2010, 140 (6) :883-899. [12] WU Y, ZHAO Q, PENG C, et al. Neutrophils promote motility of cancer cells via a hyaluronan-mediated TLR4/PI3K activation loop[J]. J Pathol, 2011, 225 (3) :438-447. [13] REISER J, BANERJEE A. Effector, memory, and dysfunctional CD8 (+) T cell fates in the antitumor immune response[J].J Immunol Res, 2016, 2016:8941260. [14] COFFELT SB, WELLENSTEIN MD, de VISSER KE. Neutrophils in cancer:Neutral no more[J]. Nat Rev Cancer, 2016, 16 (7) :431-446. [15] HA H, DEBNATH B, NEAMATI N. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases[J]. Theranostics, 2017, 7 (6) :1543-1588. [16] GALDIERO MR, BONAVITA E, BARAJON I, et al. Tumor associated macrophages and neutrophils in cancer[J]. Immunobiology, 2013, 218 (11) :1402-1410. [17] GIAMBERNARDI TA, SAKAGUCHI AY, GLUHAK J, et al. Neutrophil collagenase (MMP-8) is expressed during early development in neural crest cells as well as in adult melanoma cells[J].Matrix Biol, 2001, 20 (8) :577-587. [18] KIDANE D, CHAE WJ, CZOCHOR J, et al. Interplay between DNA repair and inflammation, and the link to cancer[J]. Crit Rev Biochem Mol Biol, 2014, 49 (2) :116-139. [19] BODOGAI M, MORITOH K, LEE-CHANG C, et al. Immunosuppressive and prometastatic functions of myeloid-derived suppressive cells rely upon education from tumor-associated B cells[J]. Cancer Res, 2015, 75 (17) :3456-3465. [20] KITAYAMA J, YASUDA K, KAWAI K, et al. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer[J]. Radiat Oncol, 2010, 5:47. [21] TAN W, SUN W, LI X, et al. Preablation neutrophil-to-lymphocyte ratio as an independent prognostic factor in locally advanced hepatocellular carcinoma patients following radiofrequency ablation[J]. J Cancer Res Ther, 2018, 14 (1) :84-89. [22] WANG WJ, ZHAO Y, XIA DD, et al. Predictive value of neutrophil-lymphocyte ratio and its dynamic change in patients with hepatocellular carcinoma undergoing transarterial chemoembolization[J]. J Clin Hepatol, 2016, 32 (6) :1139-1144. (in Chinese) 王文军, 赵艳, 夏冬东, 等.中性粒细胞与淋巴细胞比值及其动态变化对经肝动脉化疗栓塞术治疗的肝细胞癌患者预后的预测价值[J].临床肝胆病杂志, 2016, 32 (6) :1139-1144. [23] CHEN K, ZHAN MX, HU BS, et al. Combination of the neutrophil to lymphocyte ratio and the platelet to lymphocyte ratio as a useful predictor for recurrence following radiofrequency ablation of hepatocellular carcinoma[J]. Oncol Lett, 2018, 15 (1) :315-323. [24] WANG Y, ATTAR BM, FUENTES HE, et al. Evaluation of the prognostic value of platelet to lymphocyte ratio in patients with hepatocellular carcinoma[J]. J Gastrointest Oncol, 2017, 8 (6) :1065-1071. [25] XUE TC, JIA QA, GE NL, et al. The platelet-to-lymphocyte ratio predicts poor survival in patients with huge hepatocellular carcinoma that received transarterial chemoembolization[J].Tumour Biol, 2015, 36 (8) :6045-6051. [26] YANG HJ, JIANG JH, LIU QA, et al. Preoperative platelet-tolymphocyte ratio is a valuable prognostic biomarker in patients with hepatocellular carcinoma undergoing curative liver resection[J]. Tumour Biol, 2017, 39 (6) :1010428317707375. [27] EGAN K, CROWLEY D, SMYTH P, et al. Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells[J]. PLo S One, 2011, 6 (10) :e26125. [28] PALUMBO JS, TALMAGE KE, MASSARI JV, et al. Platelets and fibrin (ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells[J].Blood, 2005, 105 (1) :178-185. [29] BAUSCHKE A, ALTENDORF-HOFMANN A, MALESSA C, et al. Which factors affect the long-term survival of patients with hepatocellular carcinoma UICC stage IV?[J]. J Cancer Res Clin Oncol, 2016, 142 (12) :2593-2601. [30] CASADEI GARDINI A, MARISI G, CANALE M, et al. Radiofrequency ablation of hepatocellular carcinoma:A meta-analysis of overall survival and recurrence-free survival[J]. Onco Targets Ther, 2018, 11:6555-6567. [31] ZHOU L, LI J, AI DL, et al. Enhanced therapeutic efficacy of combined use of sorafenib and transcatheter arterial chemoembolization for treatment of advanced hepatocellular carcinoma[J]. Jpn J Clin Oncol, 2014, 44 (8) :711-717. 期刊类型引用(2)
1. 郭佳佳,张文果,王文秀,张晓丽,马徜徉. 血清Nox2、ATG7水平对新生儿窒息心肌损伤的评估价值. 安徽医药. 2024(09): 1791-1795 .  百度学术
百度学术2. 蔡卓玮,胡刚峰,章波. miR-203在乙型肝炎病毒相关肝细胞癌诊断及预后中的意义. 广西医科大学学报. 2022(11): 1773-1780 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 2149 KB)
PDF下载 ( 2149 KB)

 下载:
下载:



 百度学术
百度学术
 下载:
下载: