Expression of TM6SF2 in hepatocellular carcinoma tissue and its bioinformatics functions
-
摘要:
目的利用肿瘤数据库挖掘数据并分析TM6SF2在肝细胞癌(HCC)组织中的表达情况,同时探讨TM6SF2的生物学作用及功能。方法利用GEPIA数据库分析HCC组织中的TM6SF2基因mRNA水平的变化。利用OncoLnc做TM6SF2基因的表达水平与HCC患者生存期的相关性分析。利用cBioPortal数据库和LinkedOmics数据库分析TM6SF2在HCC组织中存在的表达相关基因。利用DAVID6. 8和STRING数据库对TM6SF2及其表达相关基因进行生物信息分析。用t检验验证HCC与癌旁组织基因mRNA表达差异。用Spearman相关系数分析基因表达的相关性。采用Kaplan-Meier生存分析计算生存率,采用广义log-rank检验估计生存率的差异。结果与正常肝组织相比,HCC组织中TM6SF2基因mRNA水平呈低表达(|log2FC|cut-off=0. 5,P <0. 01)。相比高表达的患者,TM6SF2低表达可明显降低HCC患者的总体生存时间(χ2=9. 897,P <0. 01)。数据分析显示,在HCC组织中与TM6SF2表达相关基因共49个。GO分析...
-
关键词:
- 癌,肝细胞 /
- 跨膜蛋白6超家族成员2 /
- 数据库,遗传学 /
- 信息学
Abstract:Objective To investigate the expression of TM6 SF2 in hepatocellular carcinoma (HCC) tissue and its biological functions by data mining in tumor databases. Methods The GEPIA database was applied to measure the change in the mRNA expression level of TM6 SF2 in HCC tissue, and OncoLnc was used to analyze the association of TM6 SF2 expression with the survival time of HCC patients. The cBioPortal and LinkedOmics databases were used to analyze the genes associated with the expression of TM6 SF2 in HCC tissue, and the DAVID6. 8 and STRING databases were used to perform a bioinformatics analysis of TM6 SF2 and the genes associated with its expression. The t-test was used to investigate the difference in the mRNA expression of TM6 SF2 between HCC tissue and adjacent tissue. The Spearman correlation coefficient was used to analyze the correlation of gene expression. The Kaplan-Meier method was used to calculate survival percentage, and the log-rank test was used to analyze the difference in survival percentage. Results Compared with the normal liver tissue, the HCC tissue had low mRNA expression of TM6 SF2 (| log2 FC | cut-off = 0. 5, P < 0. 01) . Compared with those with high expression of TM6 SF2, the patients with low expression had a significant reduction in overall survival time (χ2= 9. 897, P < 0. 01) . Data analysis showed that a total of 49 genes were associated with the expression of TM6 SF2 in HCC tissue, and the gene ontology analysis showed that these genes were enriched in the biological processes and functions including fatty acid synthesis, fatty acid ligase activation, and thrombin regulation (P< 0. 05) . The Kyoto Encyclopedia of Genes and Genome pathway analysis showed that these genes were mainly involved in the signaling pathways of alanine metabolism, peroxisome proliferator-activated receptor signaling pathway, and bile secretion (P < 0. 05) . The protein-protein interaction network analysis showed that the genes of SERPINC1, NR1 I2, SERPINA10, and SLC10 A1 had marked or potential interaction with TM6 SF2 (P < 0. 01) . Conclusion Tumor data mining can quickly obtain the information on the expression of TM6 SF2 in HCC tissue and provide a bioinformatics basis for exploring the role of TM6 SF2 in the development and progression of HCC.
-
原发性肝癌的发病率持续增长, 已居恶性肿瘤死亡率的第四位[1], 尽管目前肝癌治疗水平已有较大提高[2], 但其预后依然不容乐观[3]。探索肝癌侵袭转移的分子机制及寻找有效的肝癌治疗靶点仍是肝癌研究的重要内容。已有研究[4-5]表明, 中性胆固醇酯水解酶1(neutral cholesterol ester hydrolase 1, NCEH1)在侵袭性前列腺癌细胞中高表达, 抑制NCEH1的表达则可降低前列腺癌细胞的增殖、转移和侵袭能力, 提示NCEH1是一促癌基因。然而截至目前, NCEH1在肝癌中的表达及作用均尚不清楚。本研究旨在了解NCEH1在肝癌组织及细胞系中的表达情况并探讨其对肝癌细胞增殖、凋亡、转移及侵袭能力的影响。
1. 材料与方法
1.1 NCEH1基因样本
选取2013年1月—2019年6月在暨南大学附属广州红十字会医院手术治疗的32例肝癌患者标本及对应的癌旁组织。所有标本离体后立即存放在液氮罐中, 然后于-80 ℃深低温箱中保存。采用实时荧光定量PCR检测NCEH1基因的相对表达量。同时从ICGC数据库(https://daco.icgc.org)下载截至2020年9月日本人群的肝癌基因表达数据(包含240个肝癌样本和220个正常肝组织样本)作为验证组, 应用R软件(4.0.2版本)中的limma、beeswarm包对NCEH1基因表达数据进行Wilcoxon秩和检验并做散点差异图进行可视化[6]。
1.2 细胞培养
HL7702正常人肝脏细胞系和SMMC-7721、Bel-7402、HepG2和Hep3B人肝癌细胞系均购自中国科学院上海生命科学研究院生物化学与细胞生物学研究所, 293T细胞购自上海吉凯基因化学技术有限公司, 细胞置于37 ℃、5% CO2细胞培养箱中, 以含10%胎牛血清(FBS)的高糖DMEM培养基培养, 按常规进行传代。
1.3 实时荧光定量PCR
提取细胞总RNA并逆转录, 在荧光定量PCR仪(TP800, 日本TAKARA公司)上行PCR检测, 反应条件为: 95 ℃预变性15 s, 然后95 ℃变性5 s, 60 ℃退火延伸30 s, 共进行45个循环, 每次在延伸阶段读取吸光值。NCEH1引物为: 上游5′- CCTGCCGTCCTTCCTTCTTC-3′; 下游5′-CCGTGGTGCCCTGTATCATTA -3′。以管家基因GAPDH(甘油醛-3-磷酸脱氢酶)为内参基因, 引物序列为: 上游5′- TGACTTCAACAGCGACACCCA -3′; 下游5′-CACCCTGTTGCTGTAGCCAAA -3′。以2-ΔΔCT法分析各细胞系中NCEH1基因的相对于内参基因的表达水平。若肝癌组织中的NCEH1基因表达水平高于相应的癌旁组织, 则为NCEH1表达上调。
1.4 慢病毒介导的小干扰RNA(siRNA)
慢病毒质粒GV122、包装质粒pGC-LV、pHelper 1.0和pHelper2.0均购自上海吉凯基因化学技术有限公司。设计针对NCEH1基因的shRNA序列如下, 正义链: 5′-CCGGATCCAGGCAGAATTTGCATTTCTCGAGAAATGCAAATTCTGCCTGGATTTTTTG-3′, 反义链: 5′AATTCAAAAAATCCAGGCAGAATTT-GCATTTCTCGAGAAATGCAAATTCTG- CCTGGAT-3′; 阴性对照组序列为, 正义链: 5′-CCGGTTCTCCGAACGTGTCACGTTTCAA- GAGAA-CGTGACACGTTCGGAGAATTTTTG-3′, 反义链: 5′-AATTCAAAAATTCTCCGA- ACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAA-3′。上述序列由上海吉凯基因化学技术有限公司合成, 将其所形成的双链DNA分别连接到siRNA慢病毒质粒GV122, 再将上述质粒分别与pGC-LV、pHelper1.0和pHelper2.0 3个包装质粒共转染至293T细胞, 上述两种慢病毒各自感染的SMMC-7721肝癌细胞系即被分别命名为NCEH1敲减组(KD组)和阴性对照组(NC组)。采用荧光倒置显微镜(micropublisher 3.3RTV, 日本奥林帕斯公司)观察两组细胞的慢病毒感染效率, 并采用实时荧光定量PCR法检测两组细胞中NCEH1基因的相对表达水平, 以检测NCEH1基因的敲减率。
1.5 MTT实验检测肝癌细胞生长增殖能力
将KD组及NC组SMMC-7721细胞培养于6孔板中, 完全培养基重悬成细胞悬液, 选取5块96孔板, 分别标记为1、2、3、4、5 d; 每组设置3个复孔, 每孔加入100 μl细胞悬液, 每板分别铺3组细胞。从铺板后第2天开始, 培养终止前4 h加入20 μl 5 mg/ml的MTT于孔中。4 h后完全吸去培养液, 加100 μl DMSO溶解甲瓒颗粒。振荡器振荡2~5 min, 酶标仪490/570 nm检测光密度(OD)值。
1.6 Annexin V-APC单染法检测肝癌细胞凋亡率
将KD组及NC组SMMC-7721细胞培养于6孔板中, 完全培养基重悬成细胞悬液, 与上清细胞收集于同一5 ml离心管中。然后1300 r/min离心5 min, 弃上清, 4 ℃预冷的PBS洗涤细胞沉淀。1×binding buffer洗涤细胞沉淀一次, 1300 r/min离心3 min, 收集细胞, 200 μl 1×binding buffer重悬细胞沉淀, 加入10 μl Annexin V-APC染色, 室温避光10~15 min。根据细胞量, 补加400~800 μl 1×binding buffer, 上机检测。
1.7 划痕愈合实验检测肝癌细胞迁移能力
将KD组及NC组SMMC-7721细胞培养于6孔板中, 完全培养基重悬成细胞悬液, 根据细胞大小决定铺板细胞密度。置于37 ℃、5% CO2培养箱培养。第2天使用划痕仪对准96孔板的上端中央部位, 向上轻推形成划痕。使用PBS轻轻漂洗2~3遍, 加入含1% FBS血清培养基, 0 h扫板。再次于37 ℃、5% CO2培养箱培养, 根据愈合程度选择合适时间用Celigo扫板, 用Celigo分析迁移面积并计算迁移率。
1.8 Transwell转移和侵袭实验
将Transwell小室置于新的24孔板中, 上室加100 μl无FBS培养基, 37 ℃培养箱中放置1 h。将KD组及NC组SMMC-7721细胞加入每个小室, 下室内加入含30% FBS的培养基600 μl, 5% CO2、37 ℃孵育48 h。倒扣小室于吸水纸上以去除培养基, 用棉拭子轻轻移去小室内非转移细胞, 将小室置于4%多聚甲醛固定液中固定30 min后捞出, 用吸水纸吸干小室表面固定液, 滴1~2滴染色液到膜的下表面染色转移细胞1~3 min后, 将小室浸泡冲洗数次, 空气晾干。在显微镜下拍照并计算各组转移细胞数。如将Transwell小室先铺Matrigel胶再重复上述实验步骤即为Transwell侵袭实验。
1.9 伦理学审查
本研究通过暨南大学附属广州红十字会医院伦理委员会审核, 批号: 穗红院医伦审2019-028-01。所有标本采集前均已获患者知情同意。
1.10 统计学方法
应用SPSS 23.0软件进行统计学分析。符合正态分布的计量资料以x±s表示, 两组间比较采用t检验。采用配对样本比较的Wilcoxon符号秩检验对32例肝癌组织和对应癌旁中的NCEH1相对表达量进行比较, 两个独立样本比较的Wilcoxon秩和检验对ICGC数据库中肝癌和正常肝组织中的NCEH1表达量进行比较。P<0.05为差异有统计学意义。统计图采用Graphpad Prism 8.0软件(美国Graphpad软件公司)绘制。
2. 结果
2.1 肝癌组织中NCEH1的表达
实时荧光定量PCR检测结果显示, 在32例患者标本中, 有22例肝癌组织中的NCEH1基因表达上调, NCEH1基因在肝癌组织中的平均表达量高于相应的癌旁组织(Z=2.263, P=0.024)(图 1a)。下载ICGC数据库中日本人群的肝癌标本(包含240个肝癌样本, 202个正常肝组织样本), 筛选出每个样本中NCEH1基因表达量进行分析。结果显示, NCEH1基因在肝癌组织中的表达水平明显高于正常肝组织(U=18 768, P<0.001)(图 1b)。
2.2 NCEH1在肝癌细胞系中的相对表达水平
t检验结果显示, NCEH1在中等侵袭转移潜能的SMMC-7721和Bel-7402细胞系中的相对表达量较低或无侵袭转移潜能的HepG2(0.004 2±0.000 2 vs 0.002 9±0.000 4, t=4.651, P=0.01;0.003 9±0.000 3 vs 0.002 9±0.000 4, t=3.218, P=0.03)和Hep3B细胞系高(0.004 2±0.000 2 vs 0.002 7±0.000 2, t=9.496, P=0.001;0.003 9±0.000 3 vs 0.002 7±0.000 2, t=3.218, P=0.005);而NCEH1在低或无侵袭转移潜能的HepG2和Hep3B细胞系中的相对表达量较正常HL-7702肝细胞系高(0.002 9±0.000 4 vs 0.001 5±0.000 1, t=5.682, P=0.005; 0.002 7±0.000 2 vs 0.001 5±0.000 1, t=11.302, P=0.000 3)。
2.3 慢病毒的感染效率及NCEH1基因敲减效率
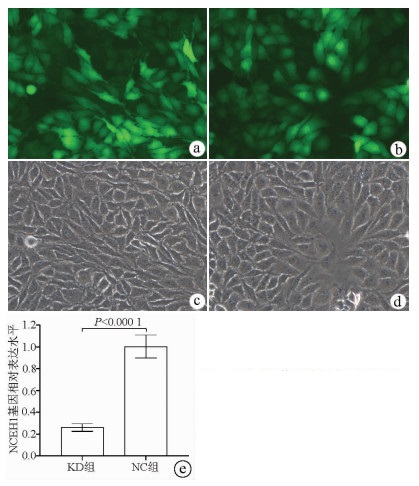
荧光显微镜观察结果显示, KD组及NC组慢病毒对肝癌细胞的感染效率均达到80%以上, KD组SMMC-7721细胞中NCEH1基因的相对表达量仅为NC组细胞的26.0%(0.260±0.035 vs 1.004±0.106, t=11.578, P=0.000 3), 即NCEH1基因在SMMC-7721人肝癌细胞系中的敲减效率达到了74.0%(图 2)。
2.4 NCEH1敲减对肝癌细胞生长增殖能力的影响
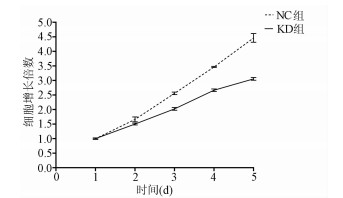
MTT检测结果显示, KD组SMMC-7721肝癌细胞生长速度较NC组明显减缓(第5天相对增殖倍数为3.055±0.046 vs 4.462±0.060, t=32.100, P=0.000 006), 提示NCEH1基因敲减明显抑制了肝癌细胞的生长增殖(图 3)。
2.5 敲减NCEH1基因对肝癌细胞凋亡的影响
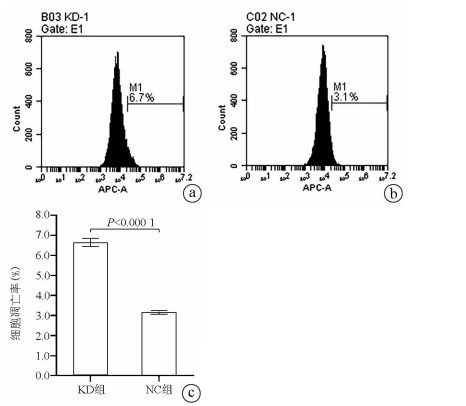
Annexin V-APC单染法流式细胞仪检测显示, KD组SMMC- 7721肝癌细胞凋亡率明显高于NC组(6.650%±0.203% vs 3.150%±0.090%, t=27.303, P=0.000 01), 提示敲减NCEH1基因可促进细胞凋亡(图 4)。
2.6 敲减NCEH1基因对肝癌细胞迁移能力的影响
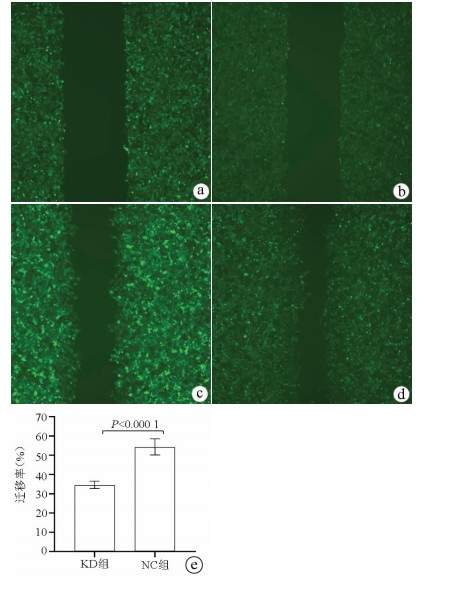
划痕愈合实验结果显示, KD组的SMMC-7721肝癌细胞迁移率明显低于NC组(34.68%±1.89% vs 54.37%±4.23%, t=9.51, P=0.000 01), 提示敲减NCEH1基因可明显抑制肝癌细胞的迁移能力(图 5)。
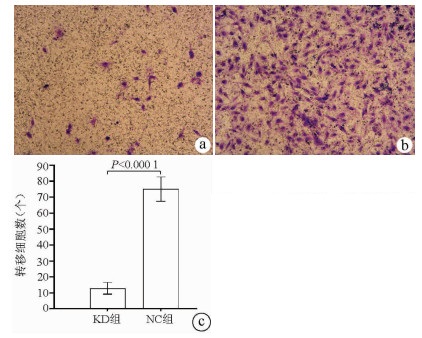
2.7 敲减NCEH1基因对肝癌细胞转移能力的影响
Transwell实验结果显示, KD组SMMC-7721肝癌细胞的转移细胞数明显低于NC组(12.89±3.63 vs 75.04±7.65, t=38.123, P<0.000 1), 提示NCEH1基因敲减可明显抑制肝癌细胞的转移能力(图 6)。
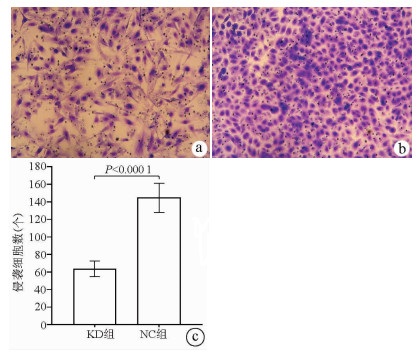
2.8 敲减NCEH1基因对肝癌细胞侵袭能力的影响
Transwell侵袭实验结果显示, KD组的SMMC-7721肝癌细胞侵袭数明显低于NC组(63.67±8.86 vs 144.52±16.60, t=22.331, P<0.000 1), 提示NCEH1基因敲减可明显抑制肝癌细胞的侵袭能力(图 7)。
3. 讨论
肝癌在全球的发病率和死亡率较高, 据估计2015年全球肝癌死亡人数约为80万[7], 而在全球每年新增肝癌病例中, 约一半发生在我国, 已成为最致命的癌症之一[8]。尽管针对肝癌的治疗手段众多, 手术切除仍然是治疗肝癌的首选方案, 但由于肝癌术后复发率相对较高, 导致肝癌术后5年无复发生存率仅为38%, 严重限制了肝癌的总体治疗效果[9-10]。因此, 探索肝癌侵袭转移的机制以获得有效的肝癌治疗靶点已成为肝癌研究的重点。
近年来的研究表明, 脂代谢异常可能是促进癌症发生发展的一个重要因素, Jiang等[11]通过蛋白质组学分析发现, 改变细胞内胆固醇的分布, 可有效抑制肝细胞癌的增殖和转移。Lin等[12]通过脂质组学分析发现, 脂质代谢的改变与肿瘤侵袭性、生长和增殖密切相关, 目前也已发现多个脂质代谢过程中的关键酶在肿瘤细胞中过表达[13-14], 这为肝癌治疗提供了一个新思路。NCEH1是一个蛋白质编码基因, 定位于人染色体3q26.31, 大小为80 970 bp。NCEH1基因编码的蛋白由408个氨基酸组成, 大小为45.808 kD, 是一种丝氨酸水解酶, 在巨噬细胞和中枢神经细胞中有较高表达[15-16], 并在调节胆固醇代谢过程中发挥重要作用[4]。已有研究[17-18]发现, NCEH1表达产物可作为乙醚脂质信号网络中连接血小板活化因子和溶血磷脂酸的中心节点, 抑制NCEH1表达产物可降低癌细胞中的醚脂质代谢并抑制肿瘤细胞迁移和生长。Chang等[5]的研究则发现, 选择性NCEH1抑制剂可明显降低人前列腺癌细胞系的迁移、侵袭、存活及增殖能力, 提示NCEH1可能与肿瘤的侵袭、迁移及增殖相关, 但NCEH1在肝癌中的表达水平及作用目前均不清楚。
为此, 本研究首先通过对本院32例肝癌及对应癌旁标本进行分析发现, NCEH1在肝癌组织中的表达明显高于对应的癌旁组织, 然后分析ICGC数据库中日本人群的肝癌基因表达数据发现, NCEH1在肝癌组织中的表达明显高于正常肝组织, 与本研究结果一致, 提示NCEH1在肝癌组织中的表达上调。继而, 本研究采用实时荧光定量PCR技术分析发现, NCEH1在所有肝癌细胞系中的表达均高于正常肝细胞系, 这与其在肝癌组织中表达上调的结果一致。更重要的是, NCEH1在侵袭转移潜能较高的肝癌细胞系中的表达明显高于低侵袭转移潜能的肝癌细胞系, 这与Chang等[5]在前列腺癌细胞系中的结果一致, 提示NCEH1与肝癌细胞的侵袭转移相关。本研究采用慢病毒介导的siRNA技术敲减NCEH1在SMMC-7721肝癌细胞系(该细胞系的NCEH1表达水平在笔者检测的4株肝癌细胞系中为最高)中的表达, 结果显示, NCEH1基因的敲减效率高达74.0%, NCEH1基因敲减的SMMC-7721肝癌细胞系得以成功建立。接下来, 本研究采用MTT生长曲线、细胞流式检测及Transwell转移和侵袭实验等一系列细胞功能实验观察NCEH1在肝癌细胞中的功能, 结果显示, NCEH1基因敲减可明显降低肝癌细胞系的增殖、转移及侵袭能力并促进凋亡, 这些结果也均与Chang等[5]在前列腺癌细胞中获得的结果一致, 提示NCEH1可能是一个潜在的肝癌治疗靶点。
对于NCEH1表达及发挥作用的分子调控机制的研究相对较少, Cheung等[19]发现在牛皮癣皮肤的皮下脂肪组织中miR-26b-5p高度上调, 而NCEH1是miR-26b-5p的直接靶点之一, miR-26b-5p可靶向下调NCEH1的表达。现有的研究显示, miR-26b-5p在不同肿瘤组织中均起着抑癌作用[20], 其在肝癌细胞系中明显下调, 且与肝癌的侵袭和转移密切相关[21-22]。而miRNA被认为可调控60%蛋白质编码基因, 并参与几乎所有细胞过程的调控[18, 23]。笔者据此推测miR-26b-5p在肝癌中也可能存在对NCEH1基因表达的负向调控, 即miR-26b-5p的下调可能促使NCEH1基因在肝癌组织中的表达上调, 并因此增强了肝癌细胞的增殖、凋亡、转移及侵袭能力。然而, NCEH1在肝癌中表达升高及发挥生物学作用的具体分子机制均尚有待于进一步研究阐明。
综上所述, 本研究结果发现, NCEH1基因在肝癌组织及细胞系中的表达明显升高, 体外实验中可以促进肝癌细胞增殖、侵袭转移并抑制凋亡, 提示其可能是一个潜在的肝癌治疗靶点。
-
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68 (6) :394-424. [2] LLOVET JM, ZUCMAN-ROSSI J, PIKARSKY E. Hepatocellular carcinoma[J]. Nat Rev Dis Primers, 2016, 2:16018. [3] LLOVET JM, MONTAL R, SIA D, et al. Molecular therapies and precision medicine for hepatocellular carcinoma[J]. Nat Rev Clin Oncol, 2018, 15 (10) :599-616. [4] MAHDESSIAN H, TAXIARCHIS A, POPOV S, et al. TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content[J]. Proc Natl Acad Sci U S A, 2014, 111 (24) :8913-8918. [5] KOZLITINA J, SMAGRIS E, STENDER S, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease[J]. Nat Genet, 2014, 46 (4) :352-356. [6] CHALASANI N, WILSON L, KLEINER DE, et al. Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with non-alcoholic fatty liver disease[J]. J Hepatol, 2008, 48 (5) :829-834. [7] KE RS, ZHANG K, LV LZ, Et al. Prognostic value and oncogene function of heterogeneous nuclear ribonucleoprotein A1overexpression in HBV-related hepatocellular carcinoma[J].Oncol Lett, 2018, 16 (3) :3746-3756. [8] ANAYA J. OncoLnc:Linking TCGA survival data to mRNAs, miRNAs, and lncRNAs[J]. Peer J Computer Science, 2016, 2:e67. [9] GAO J, AKSOY BA, DOGRUSOZ U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal[J]. Sci Signal, 2013, 6 (269) :pl1. [10] VASAIKAR SV, STRAUB P, WANG J, et al. LinkedOmics:Analyzing multi-omics data within and across 32 cancer types[J]. Nucleic Acids Res, 2017, 46 (D1) :d956-d963. [11] HUANG DW, SHERMAN BT, LEMPICKI RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources[J]. Nat Protoc, 2009, 4 (1) :44-57. [12] SZKLARCZYK D, FRANCESCHINI A, WYDER S, et al. STRING v10:Protein-protein interaction networks, integrated over the tree of life[J]. Nucleic Acids Res, 2014, 43 (D1) :d447-d452. [13] ZHANG YP, WANG F, CHEN PF, et al. Expression and significance of the ABAT gene in hepatocellular carcinoma:An analysis based on data mining[J]. J Clin Hepatol, 2019, 35 (3) :553-558. (in Chinese) 张玉鹏, 王帆, 陈鹏飞, 等.基于数据挖掘分析ABAT基因在肝细胞癌中的表达及意义[J].临床肝胆病杂志, 2019, 35 (3) :553-558. [14] ESLAM M, GEORGE J. Genetic and epigenetic mechanisms of NASH[J]. Hepatol Int, 2016, 10 (3) :394-406. [15] ESLAM M, VALENTI L, ROMEO S. Genetics and epigenetics of NAFLD and NASH:Clinical impact[J]. J Hepatol, 2018, 68 (2) :268-279. [16] ROMEO S, KOZLITINA J, XING C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease[J]. Nat Genet, 2008, 40 (12) :1461-1465. [17] DONGIOVANNI P, ROMEO S, VALENTI L. Genetic factors in the pathogenesis of nonalcoholic fatty liver and steatohepatitis[J]. Biomed Res Int, 2015, 2015:460190. [18] ANSTEE QM, SETH D, DAY CP. Genetic factors that affect risk of alcoholic and nonalcoholic fatty liver disease[J]. Gastroenterology, 2016, 150 (8) :1728-1744. e7. [19] FALLETI E, CUSSIGH A, CMET S, et al. PNPLA3 rs738409and TM6SF2 rs58542926 variants increase the risk of hepatocellular carcinoma in alcoholic cirrhosis[J]. Dig Liver Dis, 2016, 48 (1) :69-75. [20] RAKSAYOT M, CHUAYPEN N, KHLAIPHUENGSIN A, et al. Independent and additive effects of PNPLA3 and TM6SF2 polymorphisms on the development of non-B, non-C hepatocellular carcinoma[J]. J Gastroenterol, 2019, 54 (5) :427-436. [21] YANG J, TREPO E, NAHON P, et al. PNPLA3 and TM6SF2variants as risk factors of hepatocellular carcinoma across various etiologies and severity of underlying liver diseases[J]. Int J Cancer, 2019, 144 (3) :533-544. [22] MELLO T, MATEROZZI M, GALLI A. PPARs and mitochondrial metabolism:From NAFLD to HCC[J]. PPAR Res, 2016, 2016:7403230. [23] KIMURA O, KONDO Y, SHIMOSEGAWA T. PPAR could contribute to the pathogenesis of hepatocellular carcinoma[J].PPAR Res, 2012, 2012:574180. [24] DAI HH, MEI LQ. Advances in nonalcoholic fatty liver disease and hepatocellular carcinoma research[J]. J Clin Hepatol, 2012, 28 (10) :797-800. (in Chinese) 代鸿华, 梅礼强.非酒精性脂肪性肝病与肝细胞癌关系的研究进展[J].临床肝胆病杂志, 2012, 28 (10) :797-800. [25] DIEHL AM, DAY C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis[J]. N Engl J Med, 2017, 377 (21) :2063-2072. 期刊类型引用(1)
1. 李晶津,王佳玉,金泽宁. 昼夜节律对肝脏胆固醇代谢的影响. 中西医结合肝病杂志. 2022(12): 1110-1113 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 3810 KB)
PDF下载 ( 3810 KB)


 下载:
下载:






 百度学术
百度学术
 下载:
下载: