ALT≤40 U/L的HBeAg阴性慢性HBV感染者抗病毒治疗指征的无创指标分析
DOI: 10.3969/j.issn.1001-5256.2021.01.011
Noninvasive indicators of indications for antiviral therapy in HBeAg-negative chronic HBV infection patients with alanine aminotransferase ≤40 U/L
-
摘要:
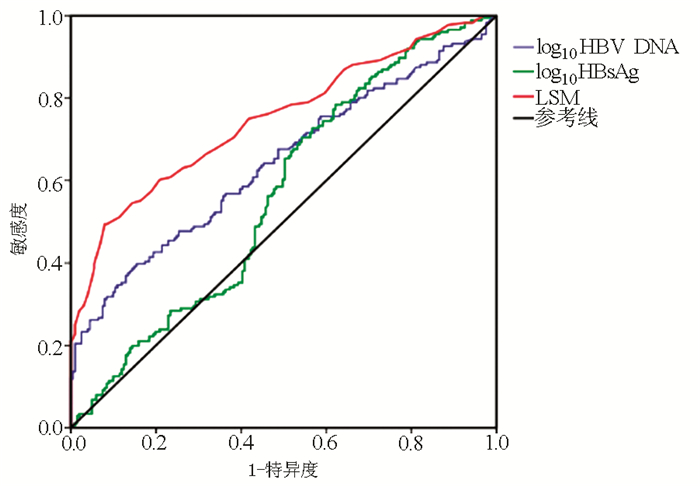
目的 在肝组织病理的指导下探索ALT≤40 U/L的HBeAg阴性慢性HBV感染者抗病毒指征的无创指标。 方法 回顾性纳入2013年10月—2018年8月延安大学附属医院收治的已行肝活检的377例ALT≤40 U/L的HBeAg阴性慢性HBV感染者,入组患者中炎症活动度<A2且纤维化分期<F2归为暂不需要抗病毒治疗组(n=266),炎症活动度≥A2,或纤维化分期≥F2归为需要抗病毒治疗组(n=111)。计数资料2组间比较采用χ2检验。满足正态分布的计量资料的2组间比较采用t检验,不满足正态分布的计量资料2组间比较采用Mann-Whitney U检验;采用二元logistic单因素分析及多因素回归分析筛选是否启用抗病毒治疗的影响因素,绘制受试者工作特征曲线(ROC曲线),应用ROC曲线评估各指标对ALT≤40 U/L的HBeAg阴性慢性HBV感染者是否需要抗病毒治疗的诊断效能。 结果 377例患者中266例(70.6%)暂不需要抗病毒治疗,111例(29.4%)伴有明显肝组织损伤,需要积极启动抗病毒治疗。多因素分析显示LSM、HBsAg、HBV DNA、Alb是启动抗病毒治疗的独立影响因素(P值均<0.05), 其HR(95%CI)分别为2.003(1.647~2.437)、1.563(1.110~2.200)、1.519(1.173~1.966)和0.939(0.884~0.998)。ROC曲线显示,LSM、HBV DNA、HBsAg预测是否需要抗病毒治疗的ROC曲线下面积(AUC)分别是0.749(0.699~0.799)、0.642(0.586~0.699)、0.565(0.507~0.623),LSM、HBV DNA、HBsAg联合诊断的AUC值更大,为0.779(0.732~0.827)。 结论 LSM、HBV DNA、HBsAg水平对ALT≤40 U/L的HBeAg阴性慢性HBV感染者启动抗病毒治疗有参考价值。 Abstract:Objective To investigate the noninvasive indicators of indications for antiviral therapy in HBeAg-negative chronic hepatitis B virus (HBV) infection patients with alanine aminotransferase (ALT) ≤40 U/L under the guidance of liver pathology. Methods A retrospective analysis was performed for the clinical data of 377 HBeAg-negative chronic HBV infection patients with ALT ≤40 U/L who were hospitalized in Affiliated Hospital of Yan'an University, from October 2013 to August 2018 and underwent liver biopsy, among whom the patients with inflammatory activity < A2 and fibrosis stage < F2 were enrolled as non-antiviral therapy group(n=266), and the patients with inflammatory activity ≥A2 or fibrosis stage ≥F2 were enrolled as antiviral therapy group(n=111). The chi-square test was used for comparison of categorical data between two groups; the t-test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups; univariate and multivariate binary logistic regression analyses were used to screen out the influencing factors for the initiation of antiviral therapy; the receiver operating characteristic (ROC) curve was plotted to evaluate the diagnostic efficiency of each indicator in determining the need for antiviral therapy in HBeAg-negative chronic HBV infection patients with ALT ≤40 U/L. Results Of all 377 patients, 266 (70.6%) did not need antiviral therapy for the time being, and 111 (29.4%) had marked liver damage and thus needed active antiviral therapy. The multivariate analysis showed that liver stiffness measurement (LSM) (odds ratio [HR]=2.003, 95% confidence interval [CI]: 1.647-2.437, P < 0.05), HBsAg (HR=1.563, 95% CI: 1.110-2.200, P < 0.05), HBV DNA (HR=1.519, 95% CI: 1.173-1.966, P < 0.05), and albumin (HR=0.939, 95% CI: 0.884-0.998, P < 0.05) were independent influencing factors for the initiation of antiviral therapy. The ROC curve analysis showed that the area under the ROC curve (AUC) was 0.749 (95% CI: 0.699-0.799) for LSM, 0.642 (95% CI: 0.586-0.699) for HBV DNA, and 0.565 (95% CI: 0.507-0.623) for HBsAg, and the combination of LSM, HBV DNA, and HBsAg had a larger AUC of 0.779 (95% CI: 0.732-0.827). Conclusion The levels of LSM, HBV DNA, and HBsAg have a reference value in determining the initiation of antiviral therapy in HBeAg-negative chronic HBV infection patients with ALT≤40 U/L. -
Key words:
- Hepatitis B Virus /
- Hepatitis B e Antigens /
- Alanine Transaminase /
- Antiviral Treatment
-
表 1 入组患者的基本特征
项目 无需抗病毒治疗组(n=266) 需抗病毒治疗组(n=111) 统计值 P值 年龄(岁) 40.18±10.08 40.69±10.97 t=-0.436 0.063 男性[例(%)] 129(48.5) 54(48.6) χ2=0.001 0.978 家族史[例(%)] 136(51.1) 60(54.1) χ2=0.269 0.604 HBV DNA(log10 IU/ml) 3.22(2.69~3.78) 2.69(3.40~4.12) Z=-1.518 0.129 HBsAg(log10 IU/ml) 3.48(2.89~3.91) 3.48(3.06~3.77) Z=-0.135 0.892 抗-HBc(S/CO) 10.31(9.20~11.54) 10.11(9.35~11.29) Z=-0.733 0.463 ALT(U/L) 31.80±32.66 34.52±31.95 t=-0.746 0.456 AST(U/L) 26.70±16.69 30.29±20.51 t=-1.773 0.077 TBil(μmol/L) 13.66±6.21 13.67±6.68 t=-0.011 0.991 Alb(g/L) 43.74±3.92 42.83±4.00 t=2.031 0.043 PLT(×109/L) 188.0(153.5~220.0) 175.0(143.0~213.0) Z=-2.060 0.039 LSM(kPa) 4.7(4.0~5.6) 6.1(4.8~7.5) Z=-6.260 <0.001 表 2 单因素和多因素分析是否需要抗病毒治疗的影响因素
项目 单因素分析 多因素分析 HR(95%CI) P值 HR(95%CI) P值 年龄(岁) 1.016(0.992~1.041) 0.191 男性 1.002(0.598~1.681) 0.993 家族史 0.973(0.599~1.580) 0.912 HBV DNA(log10 IU/ml) 1.569(1.184~2.078) 0.002 1.519(1.173~1.966) 0.002 HBsAg(log10 IU/ml) 1.672(1.171~2.387) 0.005 1.563(1.110~2.200) 0.011 抗-HBc(S/CO) 0.941(0.821~1.078) 0.381 ALT(U/L) 0.991(0.969~1.014) 0.447 AST(U/L) 1.020(0.983~1.059) 0.292 TBil(μmol/L) 1.002(0.965~1.040) 0.933 Alb(g/L) 0.944(0.887~1.005) 0.071 0.939(0.884~0.998) 0.044 PLT(×109/L) 0.998(0.993~1.002) 0.318 LSM(kPa) 1.984(1.623~2.425) <0.001 2.003(1.647~2.437) <0.001 表 3 LSM、HBV DNA、HBsAg对是否抗病毒治疗的预测价值比较
项目 LSM HBV DNA HBsAg AUC (95%CI) 0.749(0.699~0.799) 0.642(0.586~0.699) 0.565(0.507~0.623) P值 <0.001 <0.001 0.030 最佳界值 6.05 kPa 3.8 log10 IU/ml 3.27 log10 IU/ml 敏感度(%) 49.4 39.8 70.5 特异度(%) 92.0 84.6 45.8 诊断准确率(%) 70.5 62.5 58.5 Youden指数 0.414 0.244 0.163 -
[1] LOZANO R, NAGHAVI M, FOREMAN K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010[J]. Lancet, 2012, 380(9859): 2095-2128. DOI: 10.1016/S0140-6736(12)61728-0 [2] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B:A 2015 update[J]. J Clin Hepatol, 2015, 23(12): 1941-1960. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2015.12.002中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2015年更新版)[J]. 临床肝胆病杂志, 2015, 31(12): 1941-1960. DOI: 10.3969/j.issn.1001-5256.2015.12.002 [3] PAPATHEODORIDIS G, VLACHOGIANNAKOS I, CHOLONGITAS E, et al. Discontinuation of oral antivirals in chronic hepatitis B: A systematic review[J]. Hepatology, 2016, 63(5): 1481-1492. DOI: 10.1002/hep.28438 [4] TERRAULT NA, LOK ASF, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology (Baltimore, Md), 2018, 67(4): 1560-1599. DOI: 10.1002/hep.29800 [5] BERSOFF-MATCHA SJ, CAO K, JASON M, et al. Hepatitis B virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus: A review of cases reported to the U.S. Food and Drug Administration Adverse Event Reporting System[J]. Ann Intern Med, 2017, 166(11): 792-798. DOI: 10.7326/M17-0377 [6] ZHAO DF.Relationship between ALT, AST, HBeAg and serum HBV DNA content in chronic hepatitis B virus carriers[J/CD]. Chin J Clin Lab Sci(Electronic Edition), 2017, 6(1): 11-13. (in Chinese)赵冬凤. 慢性乙肝病毒携带者ALT、AST及HBeAg与血清HBV DNA含量的关系[J/CD]. 临床检验杂志(电子版), 2017, 6(1): 11-13. [7] ZHOU LL, LIU N, LI CX, et al. Current status of the diagnosis and treatment of chronic hepatitis B virus infection with immune control[J]. J Clin Hepatol, 2020, 36(5): 1134-1137. (in Chinese). DOI: 10.3969/j.issn.1001-5256.2020.05.041周路路, 刘娜, 李春霞, 等. 慢性HBV感染免疫控制期的抗病毒治疗[J]. 临床肝胆病杂志, 2020, 36(5): 1134-1137. DOI: 10.3969/j.issn.1001-5256.2020.05.041 [8] Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2019.12.007中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007 [9] LI Q, CHEN L, ZHOU Y. Diagnostic accuracy of liver stiffness measurement in chronic hepatitis B patients with normal or mildly elevated alanine transaminase levels[J]. Sci Rep, 2018, 8(1): 5224. DOI: 10.1038/s41598-018-23646-2 [10] BRAVO AA, SHETH SG, CHOPRA S. Liver biopsy[J]. N Engl J Med, 2001, 344(7): 495-500. DOI: 10.1056/NEJM200102153440706 [11] BEDOSSA P, POYNARD T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group[J]. Hepatology, 1996, 24(2): 289-293. DOI: 10.1002/hep.510240201 [12] CASTERA L, FORNS X, ALBERTI A. Non-invasive evaluation of liver fibrosis using transient elastography[J]. J Hepatol, 2008, 48(5): 835-847. DOI: 10.1016/j.jhep.2008.02.008 [13] ZHAO Y, LI YE, QI L, et al. Antiviral curative effects of tenofovir and entecavir in treatment of aged patients with chronic hepatitis B and their regulation on inflammation factors[J]. J Jilin Univ(Med Edit), 2019, 45(1): 117-122. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-BQEB201901022.htm赵阳, 李烨, 齐玲, 等. 替诺福韦酯和恩替卡韦治疗老年慢性乙型肝炎患者的抗病毒疗效及对致炎细胞因子的调节作用[J]. 吉林大学学报(医学版), 2019, 45(1): 117-122. https://www.cnki.com.cn/Article/CJFDTOTAL-BQEB201901022.htm [14] LIM SG. Influencing factors of initiation and timing of discontinuation of treatment for chronic hepatitis B[J/CD]. Chin J Exp Clin Infect Dis (Electronic Edition), 2019, 13(6):528.(in Chinese)Seng-Gee LIM. 慢性乙型肝炎治疗开始和停药时机的影响因素[J/CD]. 中华实验和临床感染病杂志(电子版), 2019, 13(6): 528. [15] DOU XG.Anti-virus treatment strategies for chronic hepatitis B virus infection with normal alanine aminotransferase[J]. Chin J Pract Intern Med, 2013, 33(6): 454-456. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-SYNK201306014.htm窦晓光. 丙氨酸转氨酶正常的慢性乙型肝炎病毒感染人群抗病毒治疗策略[J]. 中国实用内科杂志, 2013, 33(6): 454-456. https://www.cnki.com.cn/Article/CJFDTOTAL-SYNK201306014.htm [16] KUMAR M, SARIN SK, HISSAR S, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT[J]. Gastroenterology, 2008, 134(5): 1376-1384. DOI: 10.1053/j.gastro.2008.02.075 [17] WANG H, RU GQ, YAN R, et al. Histologic disease in chinese chronic hepatitis B patients with low viral loads and persistently normal alanine aminotransferase levels[J]. J Clin Gastroenterol, 2016, 50(9): 790-796. DOI: 10.1097/MCG.0000000000000544 [18] BAI XX, DONG B, GAO HY, et al. Clinical features of immune escape phase and immune control phase of chronic HBV infection[J]. J Clin Hepatol, 2018, 34(12): 2568-2571. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2018.12.012白萧萧, 东冰, 高红艳, 等. 慢性HBV感染者免疫逃逸期与免疫控制期的临床特征分析[J]. 临床肝胆病杂志, 2018, 34(12): 2568-2571. DOI: 10.3969/j.issn.1001-5256.2018.12.012 [19] LIU WR, TIAN MX, JIN L, et al. High levels of hepatitis B surface antigen are associated with poorer survival and early recurrence of hepatocellular carcinoma in patients with low hepatitis B viral loads[J]. Ann Surg Oncol, 2015, 22(3): 843-850. DOI: 10.1245/s10434-014-4043-5 [20] TSENG T C, LIU C J, YANG H C, et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load[J]. Gastroenterology, 2012, 142(5): 1140-1149. DOI: 10.1053/j.gastro.2012.02.007 [21] CHOI GH, KIM GA, CHOI J, et al. High risk of clinical events in untreated HBeAg-negative chronic hepatitis B patients with high viral load and no significant ALT elevation[J]. Aliment Pharmacol Ther, 2019, 50(2): 215-226. DOI: 10.1111/apt.15311 [22] NAKAZAWA T, SHIBUYA A, TAKEUCHI A, et al. Viral level is an indicator of long-term outcome of hepatitis B virus e antigen-negative carriers with persistently normal serum alanine aminotransferase levels[J]. J Viral Hepat, 2011, 18(7): e191-e199. http://www.ncbi.nlm.nih.gov/pubmed/21692932/ -



 PDF下载 ( 2131 KB)
PDF下载 ( 2131 KB)

 下载:
下载:


