肝细胞癌患者肝动脉化疗栓塞术后外周血长链非编码RNA TINCR水平与Th1/Th2平衡及预后的相关性分析
DOI: 10.3969/j.issn.1001-5256.2021.01.016
Association of the expression level of the long non-coding RNA TINCR in peripheral blood with Th1/Th2 balance and prognosis after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma
-
摘要:
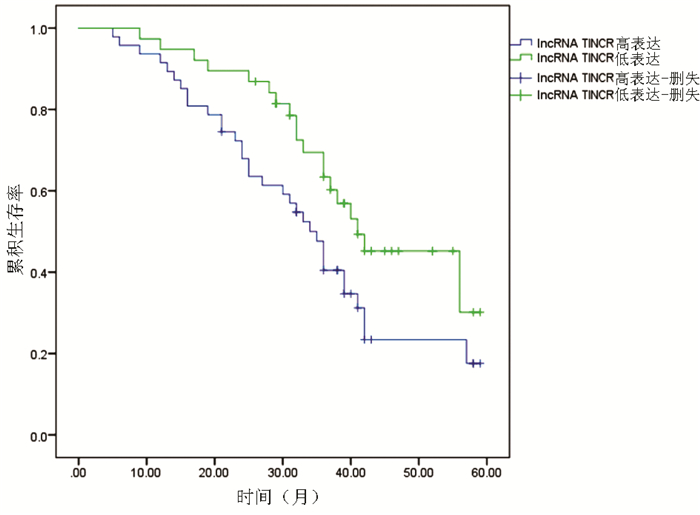
目的 探讨分析外周血长链非编码RNA(lncRNA) TINCR表达水平与肝细胞癌(HCC)患者行肝动脉化疗栓塞术(TACE)术后辅助性T淋巴细胞1(Th1)/Th2平衡及预后的相关性。 方法 回顾性选取2015年3月—2017年3月在湖北省武汉市金银潭医院行TACE治疗的HCC患者85例,并随机抽取在本院体检的健康志愿者30例作为对照组。检测HCC患者TACE完成后7 d及对照组外周血lncRNA TINCR表达情况、Th1/Th2细胞因子表达情况。计量资料组间比较采用t检验,计数资料组间比较采用χ2检验,等级资料组间比较采用Kruskal-Wallis H检验。lncRNA TINCR相对表达量与Th1/Th2细胞因子的相关性采用Spearman相关性分析。lncRNA TINCR相对表达量对生存情况的影响采用Kaplan-Meier生存曲线分析,生存曲线比较采用log-rank检验。 结果 HCC组患者外周血lncRN TINCR相对表达量(1.784±0.429)高于对照组(0.926±0.263),差异有统计学意义(t=10.277,P<0.05)。lncRNA TINCR相对表达与HCC患者血管侵犯、肿瘤大小、肿瘤分期及血清AFP水平有关(t值分别为3.958、4.499、3.361、4.949,P值分别为<0.001、<0.001、0.001、<0.001)。HCC组患者血清IFNγ、IL-2水平明显低于对照组[(13.48±5.20) pg/ml vs (27.49±7.21) pg/ml、(21.89±6.45) pg/ml vs (32.05±7.89) pg/ml,t值分别为11.408、6.986,P值均<0.05],而IL-4、IL-10水平明显高于对照组[(13.73±3.35) pg/ml vs (9.36±2.73) pg/ml、(12.75±2.74) pg/ml vs (8.93±2.16) pg/ml,t值分别为6.426、6.909,P值均<0.05]。HCC患者外周血lncRNA TINCR相对表达量与血清IFNγ、IL-2水平呈负相关(r值分别为-3.164、-3.270,P值均<0.001),与患者血清IL-4、IL-10呈正相关(r值分别为2.963、3.044,P值分别为0.007、<0.001)。以lncRNA TINCR相对表达量≥1.700作为高表达,85例HCC患者中lncRNA TINCR高表达患者47例,低表达患者38例。log-rank检验结果显示,lncRNA TINCR低表达患者生存情况明显优于高表达患者(χ2=4.182, P<0.05)。 结论 HCC患者行TACE后外周血lncRNA TINCR表达明显高于健康人群,lncRNA TINCR表达与患者病理特征及Th1/Th2免疫平衡有密切联系,可作为患者生存预后的潜在预测指标之一。 -
关键词:
- 癌, 肝细胞 /
- 化学栓塞, 治疗性 /
- RNA, 长链非编码 /
- Th1-Th2平衡 /
- 预后
Abstract:Objective To investigate the association of the expression level of the long non-coding RNA (lncRNA) TINCR in peripheral blood with Th1/Th2 balance and prognosis after transcatheter arterial chemoembolization (TACE) in patients with hepatocellular carcinoma (HCC). Methods A retrospective analysis was performed for the clinical data of 85 patients with HCC who were treated with TACE in Jinyintan Hospital from March 2015 to March 2017, and 30 healthy volunteers who underwent physical examination in our hospital were randomly selected as control group. The expression of lncRNA TINCR and Th1/Th2 cytokines in peripheral blood were measured on day 7 after TACE for HCC patients and the control group. The t-test was used for comparison of continuous data, the chi-square test was used for comparison of categorical data, and the Kruskal-Wallis H test was used for comparison of ranked data. A Spearman correlation analysis was used to investigate the correlation of the relative expression of lncRNA TINCR with Th1/Th2 cytokines. The Kaplan-Meier survival curves were used to investigate the influence of the relative expression of lncRNA TINCR on survival, and the log-rank test was used for comparison of survival curves. Results The HCC group had significantly higher relative expression of lncRNA TINCR in peripheral blood than the control group (1.784±0.429 vs 0.926±0.263, t=10.277, P < 0.05). The relative expression of lncRNA TINCR was associated with vascular invasion, tumor size, tumor stage, and serum alpha-fetoprotein (t=3.958, 4.499, 3.361, and 4.949, P < 0.001, P < 0.001, P=0.001, and P < 0.001). Compared with the control group, the HCC group had significantly lower serum levels of interferon-γ (IFNγ)[(13.48±5.20) pg/ml vs (27.49±7.21) pg/ml, t=11.408, P < 0.05] and interleukin-2 (IL-2) [(21.89±6.45) pg/ml vs (32.05±7.89) pg/ml, t=6.986, P < 0.05] and significantly higher serum levels of interleukin-4 (IL-4) [(13.73±3.35) pg/ml vs (9.36±2.73) pg/ml, t=6.426, P < 0.05] and interleukin-10 (IL-10) [(12.75±2.74) pg/ml vs (8.93±2.16) pg/ml, t=6.909, P < 0.05]. In HCC patients, the relative expression of lncRNA TINCR in peripheral blood was negatively correlated with the serum levels of IFNγ and IL-2(r=-3.164 and -3.270, both P < 0.001) and was positively correlated with the serum levels of IL-4 and IL-10 (r=2.963 and 3.044, P=0.007 and P < 0.001). With the relative expression of lncRNA TINCR of ≥1.700 as high expression, there were 47 HCC patients with high expression and 38 HCC patients with low expression. The log-rank test showed that the patients with low expression of lncRNA TINCR had significantly better survival than those with high expression (χ2=4.182, P < 0.05). Conclusion HCC patients have significantly higher expression of lncRNA TINCR in peripheral blood than the healthy population, and the expression of lncRNA TINCR is closely associated with the pathological features and Th1/Th2 immune balance of patients and can thus be used as one of the potential indicators for predicting the survival and prognosis of patients. -
由于肝细胞癌(HCC)缺少特异性的早期症状,患者临床确诊时大多处于中晚期,无法进行根治性手术,患者5年生存率不足5%[1]。近年来,随着现代影像设备的不断发展以及微创技术水平的不断提高,在大型设备数字减影机的应用下,对于无法进行手术根治的HCC患者行肝动脉化疗栓塞术(TACE)成为主要治疗方式[2]。TACE介入治疗可有效减少、阻断肿瘤血供,从而导致肿瘤缺血、缺氧而坏死[3]。长链非编码RNA(long non-coding RNA, lncRNA)是一类缺乏开放阅读框、长度>200个核苷酸的非编码RNA。近年研究[4]显示,lncRNA参与了机体各种生理以及病理过程,同时在肿瘤细胞增殖、迁移等过程中作为抑癌基因或致癌基因发挥相应的正反馈调节作用或负反馈调节作用。lncRNA TINCR是一种非典型lncRNA,其在体细胞组织分化以及肿瘤进程中均具有重要作用[5]。本研究探讨分析HCC外周血lncRNA TINCR表达水平与TACE术后辅助性T淋巴细胞1(Th1)/Th2平衡及预后相关性,为进一步揭示HCC介入治疗的预后情况提供依据。
1. 资料与方法
1.1 研究对象
回顾性选取2015年3月—2017年3月本院行TACE治疗的HCC患者。随机抽取在本院体检的健康志愿者作为对照组。纳入标准:(1)治疗前经超声、CT或2种以上影像学检查确诊为HCC;(2)经病理检查确诊为原发性肝癌;(3)凝血功能正常;(4)介入治疗术前未接受放疗或化疗治疗;(5)预计生存期>3个月;(6)TNM分期Ⅱ~Ⅲ期;(7)患者自愿签署知情同意书。排除标准:(1)腹水中至大量;(2)合并其他恶性肿瘤患者;(3)合并严重心脑血管疾病患者;(4)合并精神疾病患者;(5)哺乳期或妊娠期女性;(6)TACE治疗后1个月内并发严重感染患者;(7)合并自身免疫性疾病或免疫缺陷性疾病患者;(8)有TACE治疗禁忌证患者。
1.2 方法
1.2.1 TACE治疗方案
患者接受局部麻醉后,采用Seldinger穿刺法,经皮股动脉穿刺插管,将导管插入肿瘤供血动脉内,随后注入造影剂,进行肝动脉造影,仔细观察患者肿瘤位置、肿瘤大小以及供血情况。随后依次注入5-氟尿嘧啶(1000~12 000 mg)、比柔多星(40~60 mg)进行栓塞化疗,使用碘化油作为栓塞剂,部分患者可使用明胶海绵进行栓塞,术后给予患者抑酸、保肝、水化支持以及抗菌药物等治疗。TACE治疗每4周1次,每例患者最多进行3次。
1.2.2 外周血lncRNA TINCR表达检测
于TACE完成后7 d抽取患者清晨空腹外周静脉血,对照组受试者于体检当日抽取清晨空腹外周静脉血。室温下静置15~30 min,离心10 min(3000 r/min,4 ℃),小心吸取上清液置于试管内,再次离心10 min(2500 r/min,4 ℃),留取上清后置于-80 ℃冰箱内保存待测。取上述血浆样本,每份400 μl,采用Qiagen miRNeasy Serun/Plasma kit试剂盒使用说明书提取血浆内总RNA,并使用紫外分光光度计测量总RNA浓度,经反转录后,使用real-time PCR检测血浆中lncRNA TINCR相对表达量,TINCR上游引物5′-CCATCCCTCTGTAACCACCT-3′,下游引物5′-GTTGGGTCTAGATTCCAGCA-3′;使用Actin作为内参基因,Actin上游引物5′-ATCATGTTTGCCTAGATCAACA-3′,下游引物5′-GATCTCTTGCTAAGCGTCCA-3′。引物均由上海生工生物工程有限公司设计合成。real-time PCR反应条件为:95 ℃ 10 min;95 ℃ 10 s、60 ℃ 35 s、72 ℃ 30 s,共40个循环;72 ℃ 10 min。采用2-△△CT法表示lncRNA TINCR相对表达量。
1.2.3 Th1/Th2细胞因子检测
HCC组患者于TACE完成后7 d抽取患者清晨空腹外周静脉血,对照组受试者于体检当日抽取清晨空腹外周静脉血,离心15 min(3500 r/min,4 ℃),取上清液,采用酶联免疫吸附法(ELISA)测定血清IFNγ、IL-2、IL-4以及IL-10水平,检测试剂盒购于美国R&D公司,检测严格参照试剂盒使用说明书进行。
1.2.4 术后随访
TACE术后对患者进行随访,随访方式包括门诊随访、电话随访等,随访截止日期为2019年8月1日,以患者死亡作为随访终点事件。
1.3 伦理学审查
本研究方案经由湖北省武汉市金银潭医院伦理委员会审批,批号:伦审2017-02A。
1.4 统计学方法
采用SPSS 22.0统计学软件进行数据分析。计量资料用x±s表示,两组间比较采用t检验;计数资料组间比较采用χ2检验; 等级资料组间比较采用Kruskal-Wallis H检验。lncRNA TINCR相对表达量与Th1/Th2细胞因子的相关性采用Spearman相关性分析。lncRNA TINCR相对表达量对生存情况的影响采用Kaplan-Meier生存曲线分析,生存曲线比较采用log-rank检验。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
共纳入HCC患者85例,其中男46例、女39例,年龄29~76岁,平均(55.72±14.29)岁;Child-Pugh分级A级26例、B级53例、C级6例;血管侵犯64例;肿瘤大小>5 cm 51例、≤5 cm 34例;临床分期Ⅱ期44例、Ⅲ期41例。另选取对照组30例,其中男17例、女13例,年龄30~75岁,平均(54.28±15.20)岁。两组性别、年龄比较差异无统计学意义(P值均>0.05)。
2.2 外周血lncRNA TINCR相对表达量比较
HCC组患者外周血lncRN TINCR相对表达量(1.784±0.429)高于对照组(0.926±0.263),差异有统计学意义(t=10.277,P<0.05)。
2.3 lncRNA TINCR相对表达量与HCC患者病理特征分析
lncRNA TINCR相对表达与HCC患者血管侵犯、肿瘤大小、肿瘤分期及血清AFP水平有关(t值分别为3.958、4.499、3.361、4.949,P值均<0.05)(表 1)。
表 1 lncRNA TINCR相对表达量与HCC患者病理特征分析项目 例数 lncRNA TINCR相对表达量 统计值 P值 性别 t=1.148 0.254 男 46 1.895±0.318 女 39 1.972±0.296 年龄 t=0.798 0.427 ≥50岁 49 1.964±0.304 <50岁 36 1.912±0.287 Child-Pugh分级 Z=0.864 0.433 A级 26 1.974±0.412 B级 53 2.061±0.397 C级 6 1.875±0.332 血管侵犯 t=3.958 <0.001 有 64 2.108±0.227 无 21 1.864±0.295 肿瘤大小 t=4.499 <0.001 >5 cm 51 2.175±0.304 ≤5 cm 34 1.903±0.218 临床分期 t=3.361 0.001 Ⅱ期 44 1.764±0.275 Ⅲ期 41 1.974±0.301 肝炎病史 t=0.293 0.770 有 67 1.922±0.341 无 18 1.896±0.307 血清AFP t=4.949 <0.001 <400 ng/ml 33 1.816±0.197 ≥400 ng/ml 52 2.095±0.283 2.4 Th1/Th2细胞因子表达水平比较
HCC组患者血清IFNγ、IL-2水平明显低于对照组(t值分别为11.408、6.986,P值均<0.05),而IL-4、IL-10水平明显高于对照组(t值分别为6.426、6.909,P值均<0.05)(表 2)。
表 2 两组受试者Th1/Th2细胞因子水平比较组别 例数 IFNγ(pg/ml) IL-2(pg/ml) IL-4(pg/ml) IL-10(pg/ml) HCC组 85 13.48±5.20 21.89±6.45 13.73±3.35 12.75±2.74 对照组 30 27.49±7.21 32.05±7.89 9.36±2.73 8.93±2.16 t值 11.408 6.986 6.426 6.909 P值 <0.05 <0.05 <0.05 <0.05 2.5 HCC患者lncRNA TINCR相对表达量与Th1/Th2细胞因子的相关性分析
HCC患者外周血lncRNA TINCR相对表达量与血清IFNγ、IL-2水平呈负相关(r值分别为-3.164、-3.270,P值均<0.001),与患者血清IL-4、IL-10呈正相关(r值分别为2.963、3.044,P值分别为0.007、<0.001)。
2.6 lncRNA TINCR相对表达量与患者预后关系
以lncRNA TINCR相对表达量≥1.700作为高表达,85例HCC患者中lncRNA TINCR高表达患者47例,低表达患者38例。log-rank检验结果显示,lncRNA TINCR低表达患者生存情况明显优于高表达患者(χ2=4.182, P<0.05)(图 1)。
3. 讨论
HCC血供75%~80%均来自于肝动脉,TACE介入治疗可有效阻断HCC供血动脉,导致肿瘤组织缺血、缺氧而发生坏死,尤其对于中晚期HCC患者而言,由于癌细胞发生扩散,多合并淋巴结转移或远处转移,TACE是目前首选治疗方案,但患者5年生存率仍然较低[6-7]。lncRNA目前已被证实在人体染色体重组、修饰过程中具有着重要的调控作用,可诱导组蛋白修饰,进行RNA选择性剪切,在基因启动子干扰以及蛋白质活性中发挥着重要作用,同时可作为肿瘤生物标志物,在肿瘤发生发展的诊断方面有重要价值[8-9]。研究[10]显示,TINCR在膀胱癌组织中表达上调,促进了膀胱癌的发生及发展,此外,抑制TINCR表达可抑制肿瘤细胞增殖而促进细胞体外凋亡,认为TINCR是膀胱癌治疗的潜在靶点。同时,研究[11]显示,在HCC组织中可出现TINCR的异常表达,并认为该种异常表达与患者临床病理特征有密切关系。
本研究选取TACE治疗的HCC患者作为研究对象,分析患者外周血lncRNA TINCR表达情况及其与患者术后Th1/Th2平衡和预后相关性。研究结果显示,与正常对照组相比较,HCC患者TACE术后仍存在lncRNA TINCR的高表达,且表达情况与患者血管侵犯、肿瘤大小、肿瘤分期及血清AFP水平有关,与相关研究[12-13]结果一致,提示HCC患者的lncRNA TINCR高表达与HCC的疾病进展程度有关,进一步证实了lncRNA TINCR与HCC的发生发展具有着密切的联系。
T淋巴细胞所介导的细胞免疫在机体排斥肿瘤过程中发挥着重要作用,Th1/Th2极化作为机体免疫应答的重要环节,对肿瘤发生发展有重要影响[14-15]。Th1细胞可分泌IFNγ、IL-2等细胞因子,参与抗肿瘤细胞免疫过程;Th2细胞主要分泌IL-4、IL-10等细胞因子,参与体液免疫过程,同时具有抗Th细胞作用[16]。在正常机体内,Th1/Th2细胞处于平衡状态,在恶性肿瘤患者中表现为Th1/Th2平衡破坏,出现Th1/Th2漂移。本研究结果显示,HCC组患者血清IFNγ、IL-2水平明显低于对照组,而IL-4、IL-10水平明显高于对照组,提示与健康人群相比较,HCC患者表现为Th1细胞功能减弱以及Th2细胞功能升高,患者Th1/Th2细胞平衡以Th2作为优势细胞,与相关研究[17-18]结果一致,这种免疫功能的改变被认为可能与肿瘤的发展有密切联系。
分析患者lncRNA TINCR表达与Th1/Th2细胞平衡之间的相关性,结果显示,HCC患者外周血lncRNA TINCR相对表达量与患者血清IFNγ、IL-2呈负相关,与患者血清IL-4、IL-10呈正相关,提示lncRNA TINCR与肿瘤的免疫调节有关,可能是TINCR调控肿瘤进展的机制之一[19-20],但仍需要进行进一步的研究证实。此外,分析lncRNA TINCR表达对患者TACE治疗后生存情况的影响,结果显示,lncRNA TINCR低表达患者生存情况显著优于高表达患者,提示lncRNA TINCR与HCC患者治疗预后密切相关,可作为患者预后预测的潜在指标[21]。关于lncRNA TINCR对HCC的影响,可能考虑lncRNA TINCR对患者机体免疫功能的调控,但是关于其具体的分子机制尚未阐明。
综上所述,HCC血管介入治疗患者术后外周血lncRNA TINCR表达明显高于健康人群,而lncRNA TINCR表达与患者病理特征及Th1/Th2免疫平衡有密切联系,可作为患者生存预后的潜在预测指标之一,值得进一步深入研究分析。
-
表 1 lncRNA TINCR相对表达量与HCC患者病理特征分析
项目 例数 lncRNA TINCR相对表达量 统计值 P值 性别 t=1.148 0.254 男 46 1.895±0.318 女 39 1.972±0.296 年龄 t=0.798 0.427 ≥50岁 49 1.964±0.304 <50岁 36 1.912±0.287 Child-Pugh分级 Z=0.864 0.433 A级 26 1.974±0.412 B级 53 2.061±0.397 C级 6 1.875±0.332 血管侵犯 t=3.958 <0.001 有 64 2.108±0.227 无 21 1.864±0.295 肿瘤大小 t=4.499 <0.001 >5 cm 51 2.175±0.304 ≤5 cm 34 1.903±0.218 临床分期 t=3.361 0.001 Ⅱ期 44 1.764±0.275 Ⅲ期 41 1.974±0.301 肝炎病史 t=0.293 0.770 有 67 1.922±0.341 无 18 1.896±0.307 血清AFP t=4.949 <0.001 <400 ng/ml 33 1.816±0.197 ≥400 ng/ml 52 2.095±0.283 表 2 两组受试者Th1/Th2细胞因子水平比较
组别 例数 IFNγ(pg/ml) IL-2(pg/ml) IL-4(pg/ml) IL-10(pg/ml) HCC组 85 13.48±5.20 21.89±6.45 13.73±3.35 12.75±2.74 对照组 30 27.49±7.21 32.05±7.89 9.36±2.73 8.93±2.16 t值 11.408 6.986 6.426 6.909 P值 <0.05 <0.05 <0.05 <0.05 -
[1] LI XC, WANG HW, LI CX. Present situation and prospect of comprehensive treatment of hepatocellular carcinoma[J]. Chin J Dig Surg, 2018, 17(5): 433-436. (in Chinese) DOI: 10.3760/cma.j.issn.1673-9752.2018.05.004李相成, 王宏伟, 李长贤. 肝癌综合治疗的现状与展望[J]. 中华消化外科杂志, 2018, 17(5): 433-436. DOI: 10.3760/cma.j.issn.1673-9752.2018.05.004 [2] Bureau of Medical Administration, National Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2020.02.007中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗规范(2019年版)[J]. 临床肝胆病杂志, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007 [3] CHEN Z, YAO L, LIU Y, et al. LncTIC1 interacts with β-catenin to drive liver TIC self-renewal and liver tumorigenesis[J]. Cancer Lett, 2018, 430: 88-96. DOI: 10.1016/j.canlet.2018.05.023 [4] HUANG LN, LENG KM, XU Y, et al. Expressions and roles of long non-coding RNA ROR in tumors[J]. Mod Med J, 2017, 45(12): 1865-1870.(in Chinese) DOI: 10.3969/j.1671-7562.2017.12.041黄立宁, 冷开明, 徐艺, 等. 长链非编码RNA ROR在肿瘤中的表达和作用[J]. 现代医学, 2017, 45(12): 1865-1870. DOI: 10.3969/j.1671-7562.2017.12.041 [5] HE T, ZHANG L, KONG Y, et al. Long non-coding RNA CASC15 is upregulated in hepatocellular carcinoma and facilitates hepatocarcinogenesis[J]. Int J Oncol, 2017, 51(6): 1722-1730. DOI: 10.3892/ijo.2017.4175 [6] CHEN Z, LIU Y, YAO L, et al. The long noncoding RNA lncZic2 drives the self-renewal of liver tumor-initiating cells via the protein kinase C substrates MARCKS and MARCKSL1[J]. J Biol Chem, 2018, 293(21): 7982-7992. DOI: 10.1074/jbc.RA117.001321 [7] TANG GJ, TIAN Y, WANG JT, et al. Advances in long non-coding RNA in chemotherapeutic resistance of hepatocellular carcinoma[J]. Med J Chin PLA, 2020, 45(5): 547-553. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-JFJY202005016.htm唐贵菊, 田塬, 王继婷, 等. 长链非编码RNA在肝癌化疗耐药中的研究进展[J]. 解放军医学杂志, 2020, 45(5): 547-553. https://www.cnki.com.cn/Article/CJFDTOTAL-JFJY202005016.htm [8] LIU T, YANG H, FAN W, et al. Mechanisms of MAFG dysregulation in cholestatic liver injury and development of liver cancer[J]. Gastroenterology, 2018, 155(2): 557-571. e14. DOI: 10.1053/j.gastro.2018.04.032 [9] CAI C, YANG L, TANG Y, et al. Prediction of overall survival in gastric cancer using a nine-lncRNA[J]. DNA Cell Biol, 2019, 38(9): 1005-1012. DOI: 10.1089/dna.2019.4832 [10] LIU HR, LIU XL, YIN BB, et al. Association between SALL4 and lncRNA in human hepatocellular carcinoma[J]. Chin J Cancer Prev Treat, 2017, 24(21): 1518-1522, 1529.(in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-QLZL201721007.htm刘海荣, 刘晓琳, 殷蓓蓓, 等. 原发性肝细胞肝癌组织SALL4与LncRNA表达相关性研究[J]. 中华肿瘤防治杂志, 2017, 24(21): 1518-1522, 1529. https://www.cnki.com.cn/Article/CJFDTOTAL-QLZL201721007.htm [11] WU J, LI JT, WEI HL, et al. Advances in the role of microRNA in the intervention of malignant transformation of precancerous lesions of the liver by regulating the activation of hepatic stellate cells[J]. J Clin Hepatol, 2020, 36(7): 1650-1654. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2020.07.045吴杰, 李京涛, 魏海梁, 等. microRNA调控肝星状细胞活化干预肝癌癌前病变的研究进展[J]. 临床肝胆病杂志, 2020, 36(7): 1650-1654. DOI: 10.3969/j.issn.1001-5256.2020.07.045 [12] PANG Y, KARTSONAKI C, TURNBULL I, et al. Diabetes, plasma glucose, and incidence of fatty liver, cirrhosis, and liver cancer: A prospective study of 0.5 million people[J]. Hepatology, 2018, 68(4): 1308-1318. DOI: 10.1002/hep.30083 [13] ZHAI JJ, DU XR, LI CX. Effect of lncRNA HOTAIR on the radiosensitivity of HCCLM3 cells[J]. Natl Med J China, 2020, 100(18): 1419-1425.(in Chinese) DOI: 10.3760/cma.j.cn112137-20190928-02130翟金俊, 杜贤荣, 李彩霞. lncRNA HOTAIR对肝癌细胞HCCLM3放射敏感性的影响[J]. 中华医学杂志, 2020, 100(18): 1419-1425. DOI: 10.3760/cma.j.cn112137-20190928-02130 [14] FANG T, LV H, LV G, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer[J]. Nat Commun, 2018, 9(1): 191. DOI: 10.1038/s41467-017-02583-0 [15] MALKI A, EL-SHARKAWY A, EL SYAED M, et al. Antitumor activities of the novel isosteviol derivative 10C against liver cancer[J]. Anticancer Res, 2017, 37(4): 1591-1601. DOI: 10.21873/anticanres.11489 [16] YANG PJ, LI X, DOU KF. Research progress of long non coding RNA in transplantation immunity[J]. Ogran Transplantation, 2018, 9(2): 91-96. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-QGYZ201802001.htm杨佩军, 李霄, 窦科峰. 长链非编码RNA在移植免疫中的研究进展[J]. 器官移植, 2018, 9(2): 91-96. https://www.cnki.com.cn/Article/CJFDTOTAL-QGYZ201802001.htm [17] CHEN SS, CHEN X, JIANG QY, et al. Expression and prognostic significance of long non-coding RNA CCAT1 in hepatocellular carcinoma[J]. J Med Res, 2018, 47(9): 86-89.(in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-YXYZ201809022.htm陈思思, 陈霞, 蒋琼英, 等. 长链非编码RNA CCAT1在肝细胞肝癌中的表达及预后意义[J]. 医学研究杂志, 2018, 47(9): 86-89. https://www.cnki.com.cn/Article/CJFDTOTAL-YXYZ201809022.htm [18] ZHANG S, ZHU J, WU XB, et al. Expressions of LncRNA TINCR and ANRIL in liver cancer tissues and their relationships with the prognosis of the patients[J]. J Trop Med, 2019, 19(1): 55-59. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-RDYZ201901014.htm张帅, 朱佳, 吴相柏, 等. 肝癌组织LncRNA TINCR和ANRIL表达水平及其与患者预后的关系[J]. 热带医学杂志, 2019, 19(1): 55-59. https://www.cnki.com.cn/Article/CJFDTOTAL-RDYZ201901014.htm [19] ZHOU W, ZHANG S, LI J, et al. lncRNA TINCR participates in ALA-PDT-induced apoptosis and autophagy in cutaneous squamous cell carcinoma[J]. J Cell Biochem, 2019, 120(8): 13893-13902. DOI: 10.1002/jcb.28662 [20] HOU A, ZHANG Y, ZHENG Y, et al. LncRNA terminal differentiation-induced ncRNA (TINCR) sponges miR-302 to upregulate cyclin D1 in cervical squamous cell carcinoma (CSCC)[J]. Hum Cell, 2019, 32(4): 515-521. DOI: 10.1007/s13577-019-00268-y [21] HE X, LI L, FANG Y, et al. In vivo imaging of leucine aminopeptidase activity in drug-induced liver injury and liver cancer via a near-infrared fluorescent probe[J]. Chem Sci, 2017, 8(5): 3479-3483. DOI: 10.1039/C6SC05712H 期刊类型引用(8)
1. 肖雷,蒋亚新,许家亮,李海春. Th1/Th2细胞因子与原发性肝癌腹腔镜手术治疗预后的关系. 河南医学研究. 2024(07): 1235-1238 .  百度学术
百度学术2. 薛蓉,尚新芳,郭雪娟,张林渊,张赟. 乳腺癌化疗患者PICC导管相关性血流感染外周血Th1/Th2及其细胞因子水平变化. 中华医院感染学杂志. 2024(10): 1526-1529 .  百度学术
百度学术3. 苏凡,曾彬,于艳艳,缪爱梅,李世芹,梁晓燕. 儿童肺炎支原体肺炎外周血γ干扰素、白细胞介素4、辅助性T细胞17/调节性T细胞水平变化及其与预后相关性. 临床军医杂志. 2024(11): 1198-1201+1204 .  百度学术
百度学术4. 张燕燕,江龙龙,赵春燕,尹伦财,卿松. 血清miR-29b、miR-371表达与中晚期肝细胞癌患者Th1/Th2平衡、临床病理特征及预后的关系. 国际检验医学杂志. 2023(09): 1064-1069 .  百度学术
百度学术5. 马海燕,冯海红,郭飞,武雪亮. 结直肠癌患者血清总蛋白、白蛋白、血红蛋白与Th1/Th2免疫平衡及调节性T细胞、辅助性T细胞17相关性分析. 中国医药导报. 2023(11): 106-110+122 .  百度学术
百度学术6. 高晓杰,陈娟,张静,郝建雯. 丁沉扶正汤对消化系统恶性肿瘤术后气滞血瘀型胃肠功能紊乱的疗效和Th1/Th2免疫漂移的影响. 中国医药导报. 2022(05): 119-123 .  百度学术
百度学术7. 闫伟,戴雪娥,郉晓宇,陈韭铭. 肝癌患者TACE治疗前后血清免疫炎症相关因子的变化及意义. 中国肿瘤临床与康复. 2022(03): 269-273 .  百度学术
百度学术8. 邸翔,吴小磊,刘伟然. 小儿肺热咳喘颗粒联合丙卡特罗治疗儿童咳嗽变异性哮喘疗效及对患儿肺功能、辅助性T细胞水平影响. 创伤与急危重病医学. 2022(06): 419-422+425 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 2128 KB)
PDF下载 ( 2128 KB)

 下载:
下载:

 下载:
下载:
 百度学术
百度学术