结节性硬化基因1/2突变与肝细胞癌进展及预后的关系
DOI: 10.3969/j.issn.1001-5256.2021.01.017
利益冲突声明:本研究不存在研究者、伦理委员会成员、受试者监护人以及与公开研究成果有关的利益冲突,特此声明。
作者贡献声明:张勇负责课题设计,资料分析,撰写论文;毛正发负责收集数据,修改论文;张勇、毛正发负责拟定写作思路,指导撰写文章并最后定稿。
Association of tuberous sclerosis gene 1/2 mutations with the progression of hepatocellular carcinoma and prognosis
-
摘要:
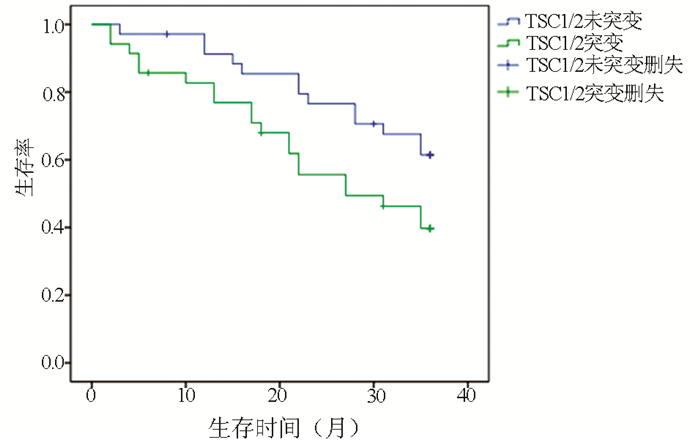
目的 探讨肝细胞癌(HCC)患者结节性硬化基因1/2(TSC1/2)突变与HCC严重程度及预后的关系,为HCC的诊断及治疗提供可行性依据。 方法 选取2012年1月—2020年1月江苏大学附属医院收治的HCC患者492例,其中59例患者出现TSC1/2基因突变(TSC1突变20例,TSC2突变41例,共同突变2例),分析TSC1/2突变组患者的临床特征,及TSC1/2突变与HCC临床分期相关性。对35例突变组和35例未突变组患者进行3年随访,观察TSC1/2突变对HCC预后的影响。计数资料两组间比较采用χ2检验;等级资料两组间比较采用Kruskal-Wallis H秩和检验;采用多元logistic回归分析相关性,随访资料采用Kaplan-Meier生存分析。 结果 492例HCC患者中,TSC1/2基因总体突变率为11.99%。TSC1、TSC2基因突变组患者性别、年龄、Child-Pugh评分及肿瘤大小与未突变组相较差异均无统计学意义(P值均>0.05),两组肿瘤个数、肝外转移情况及PS评分比较差异均有统计学意义(P值均<0.05)。logistic回归分析结果显示,TSC1/TSC2基因突变与HCC临床分期严重程度呈正相关(OR=1.706,P<0.05)。随访结果显示,TSC1/2突变组患者的生存率明显低于未突变组,前者3年病死率高达60.3%,与未突变组(38.6%)比较差异有统计学意义(χ2=3.923, P<0.05)。 结论 TSC1/2基因突变可能早期预测HCC的恶性进展,TSC1/2突变的患者预后更差,应用基因突变靶向药物治疗对于延缓HCC发展可能具有一定疗效。 Abstract:Objective To investigate the association of tuberous sclerosis gene 1/2 (TSC1/2) mutation with disease severity and prognosis in patients with hepatocellular carcinoma (HCC), and to provide a feasible basis for the diagnosis and treatment of HCC. Methods A total of 492 patients with HCC who were admitted to The Affiliated Hospital of Jiangsu University from January 2012 to January 2020 were enrolled, among whom 59 had TSC1/2 mutations (20 with TSC1 mutations, 41 with TSC2 mutations, and 2 had both TSC1 and TSC2 mutations). The clinical features of patients with TSC1/2 mutations were analyzed, and the association of TSC1/2 mutations with the clinical stage of HCC was analyzed. The 35 patients in the mutation group and 35 in the non-mutation group were followed up for 3 years to observe the effect of TSC1/2 mutations on the prognosis of HCC. The chi-square test was used for comparison of categorical data between groups; the Kruskal-Wallis H test was used for comparison of ranked data between groups; a multivariate logistic regression analysis was used to investigate association; the Kaplan-Meier survival analysis was used to analyze follow-up data. Results For the 492 patients with HCC, the overall TSC1/2 mutation rate was 11.99%. There were no significant differences in sex, age, Child score, and tumor size between the TSC1/TSC2 mutation group and the non-mutation group (all P > 0.05), while there were significant differences in tumor number, extrahepatic metastasis, and PS score between the two groups (all P < 0.05). The logistic regression analysis showed that TSC1/TSC2 gene mutation was positively correlated with the severity of HCC (odds ratio=1.706, P < 0.05). The follow-up results showed that the TSC1/2 mutation group had a significantly lower survival rate than the non-mutation group, and there was a significant difference in 3-year mortality rate between the TSC1/2 mutation group and the non-mutation group (60.3% vs 38.6%, χ2=3.923, P < 0.05). Conclusion TSC1/TSC2 gene mutation may predict the malignant progression of HCC in the early stage, and patients with TSC1/2 mutation tend to have poor prognosis. Targeted drug therapy for gene mutations may have a certain effect in delaying the progression of HCC. -
Key words:
- Carcinoma, Hepatocellular /
- Tuberous Sclerosis /
- Gene /
- Mutation
-
表 1 TSC1/2基因突变患者临床特征比较
项目 TSC1 TSC2 突变(n=20) 未突变(n=472) χ2值 P值 突变(n=41) 未突变(n=451) χ2值 P值 性别[例(%)] 0.80 >0.05 1.08 >0.05 男 14(70) 344(73) 27(66) 331(73) 女 6(30) 128(27) 14(34) 120(27) 年龄[例(%)] 0.01 >0.05 0.12 >0.05 40~60岁 12(60) 284(61) 25(61) 271(60) 60~75岁 8(40) 188(39) 16(39) 180(40) 肿瘤大小[例(%)] 1.92 >0.05 0.25 >0.05 ≥5个 13(65) 309(65) 26(63) 296(66) <5个 7(35) 163(35) 15(37) 155(34) 肿瘤个数[例(%)] 29.90 <0.05 47.5 <0.05 单个 1(05) 130(28) 5(12) 126(28) 多个 19(95) 342(72) 36(88) 325(72) 肝外转移[例(%)] 10.91 <0.05 7.56 <0.05 否 7(35) 394(83) 17(41) 384(85) 是 13(65) 78(17) 24(59) 67(15) PS评分[例(%)] 4.58 <0.05 4.19 <0.05 0~2分 14(70) 410(87) 31(76) 393(87) 3~4分 6(30) 62(13) 10(24) 58(13) Child-Pugh分级[例(%)] 4.39 >0.05 2.51 >0.05 A级 1(5) 100(22) 8(10) 93(20) B级 14(70) 309(65) 24(68) 299(67) C级 5(25) 63(13) 9(22) 59(13) 表 2 随访的TSC1/2突变组与未突变组患者基本情况比较
项目 TSC1/2未突变(n=35) TSC1/2突变(n=35) χ2值 P值 性别[例(%)] 2.20 >0.05 男 25(71) 19(54) 女 10(29) 16(46) 年龄[例(%)] 1.43 >0.05 ≥40岁且<60岁 21(60) 16(46) ≥60岁且<75岁 14(40) 19(54) 一级亲属[例(%)] 1.29 >0.05 患肝癌 6(17) 10(29) 无肝癌 29(83) 25(71) 二级亲属[例(%)] 1.75 >0.05 患肝癌 5(14) 2(6) 无肝癌 30(86) 33(94) 吸烟史[例(%)] 0.58 >0.05 有 20(57) 19(54) 无 15(43) 16(46) 饮酒史[例(%)] 0.01 >0.05 有 27(77) 23(66) 无 8(23) 12(34) 治疗方式[例(%)] 0.86 >0.05 手术治疗 12(34) 15(43) 介入治疗 18(51) 17(49) 药物治疗 5(15) 3(8) 肝病病程[例(%)] 2.69 >0.05 ≤10年 6(17) 12(34) >10年 29(83) 23(66) -
[1] BUDNY A, KOZŁOWSKI P, KAMIN'SKA M, et al. Epidemiology and risk factors of hepatocellular carcinoma[J]. Pol Merkur Lekarski, 2017, 43(255): 133-139. [2] ZHU AX, WALLNER KE, FRIVOLD GP, et al. Prostate brachytherapy seed migration to the right coronary artery associated with an acute myocardial infarction[J]. Brachytherapy, 2006, 5(4): 262-265. DOI: 10.1016/j.brachy.2006.08.004 [3] CROCETTI L, BARGELLINI I, CIONI R. Loco-regional treatment of HCC: Current status[J]. Clin Radiol, 2017, 72(8): 626-635. DOI: 10.1016/j.crad.2017.01.013 [4] ALGHAMDI MA, LEE YR, SWIHA M, et al. The effect of sorafenib (S) starting dose and dose intensity on survival in patients with advanced hepatocellular carcinoma (HCC)[J]. J Clin Oncol, 2017, 9(14): 4918-4928. [5] KAPLAN DE, YU S, TADDEI TH, et al. Up-titration of sorafenib for hepatocellular carcinoma: Impact on duration of exposure and cost[J]. J Clin Oncol, 2017, 35: 385. [6] CLARK JW, EDER JP, RYAN D, et al. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors[J]. Clin Cancer Res, 2005, 11(15): 5472-5480. DOI: 10.1158/1078-0432.CCR-04-2658 [7] STRUMBERG D, AWADA A, HIRTE H, et al. Pooled safety analysis of BAY 43-9006 (sorafenib) monotherapy in patients with advanced solid tumours: Is rash associated with treatment outcome?[J]. Eur J Cancer, 2006, 42(4): 548-556. DOI: 10.1016/j.ejca.2005.11.014 [8] ZUCMAN-ROSSI J, VILLANUEVA A, NAULT JC, et al. Genetic landscape and biomarkers of hepatocellular carcinoma[J]. Gastroenterology, 2015, 149(5): 1226-1239.e4. DOI: 10.1053/j.gastro.2015.05.061 [9] JOZWIAK J, JOZWIAK S, WLODARSKI P. Possible mechanisms of disease development in tuberous sclerosis[J]. Lancet Oncol, 2008, 9(1): 73-79. DOI: 10.1016/S1470-2045(07)70411-4 [10] NORTHRUP H, KRUEGER DA, International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: Recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference[J]. Pediatr Neurol, 2013, 49(4): 243-254. DOI: 10.1016/j.pediatrneurol.2013.08.001 [11] LI SQ, LI HZ, ZHANG YS. Clinical analysis of spontaneous rupture in tuberous sclerosis-related renal angiomyolipoma[J]. J Clin Urol, 2014, 29(12): 1070-1072. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-LCMW201412010.htm李书强, 李汉忠, 张玉石. 结节性硬化症相关肾错构瘤自发性破裂出血临床诊治分析[J]. 临床泌尿外科杂志, 2014, 29(12): 1070-1072. https://www.cnki.com.cn/Article/CJFDTOTAL-LCMW201412010.htm [12] XU B, ZHANG Q, JIN J. Laparoscopic aspiration for central renal angiomyolipoma: A novel technique based on single-center initial experience[J]. Urology, 2013, 81(2): 313-318. DOI: 10.1016/j.urology.2012.09.047 [13] HO DWH, CHAN LK, CHIU YT, et al. TSC1/2 mutations define a molecular subset of HCC with aggressive behaviour and treatment implication[J]. Gut, 2017, 66(8): 1496-1506. DOI: 10.1136/gutjnl-2016-312734 [14] Chinese Society of Clinical Oncology. CSCO guidelines for diagnosis and treatment of hepatocellular carcinoma(2020)[M]. Beijing: China People's Health Press, 2020: 28-29. (in Chinese)中国临床肿瘤学会. 原发性肝癌诊疗指南2020[M]. 北京:中国人民卫生出版社, 2020: 28-29. [15] CALDERARO J, COUCHY G, IMBEAUD S, et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification[J]. J Hepatol, 2017, 67(4): 727-738. DOI: 10.1016/j.jhep.2017.05.014 [16] NISHIDA N, KUDO M. Oncogenic signal and tumor microenvironment in hepatocellular carcinoma[J]. Oncology, 2017, 93(Suppl 1): 160-164. [17] DIBBLE CC, MANNING BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output[J]. Nat Cell Biol, 2013, 15(6): 555-564. DOI: 10.1038/ncb2763 [18] HOWELL JJ, RICOULT SJ, BEN-SAHRA I, et al. A growing role for mTOR in promoting anabolic metabolism[J]. Biochem Soc Trans, 2013, 41(4): 906-912. DOI: 10.1042/BST20130041 [19] European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16[J]. Cell, 1993, 75(7): 1305-1315. DOI: 10.1016/0092-8674(93)90618-Z [20] CRINO PB, NATHANSON KL, HENSKE EP. The tuberous sclerosis complex[J]. N Engl J Med, 2006, 355(13): 1345-1356. DOI: 10.1056/NEJMra055323 [21] NABBOUT R, BELOUSOVA E, BENEDIK MP, et al. Epilepsy in tuberous sclerosis complex: Findings from the TOSCA Study[J]. Epilepsia Open, 2019, 4(1): 73-84. DOI: 10.1002/epi4.12286 [22] JANSEN AC, BELOUSOVA E, BENEDIK MP, et al. Clinical characteristics of subependymal giant cell astrocytoma in tuberous sclerosis complex[J]. Front Neurol, 2019, 10: 705. DOI: 10.3389/fneur.2019.00705 -



 PDF下载 ( 2115 KB)
PDF下载 ( 2115 KB)

 下载:
下载:


