丹参酮Ⅰ在肝缺血再灌注损伤小鼠模型中的保护作用
DOI: 10.3969/j.issn.1001-5256.2021.01.021
Protective effect of tanshinone I in a mouse model of hepatic ischemia-reperfusion injury
-
摘要:
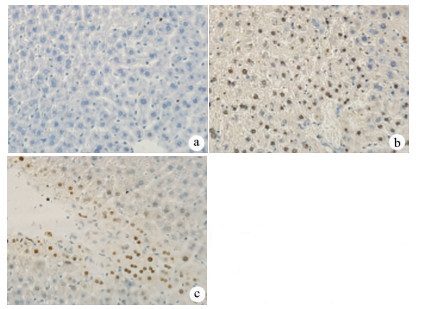
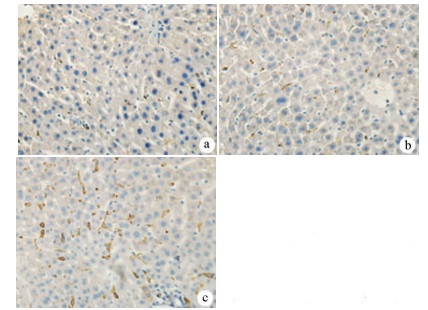
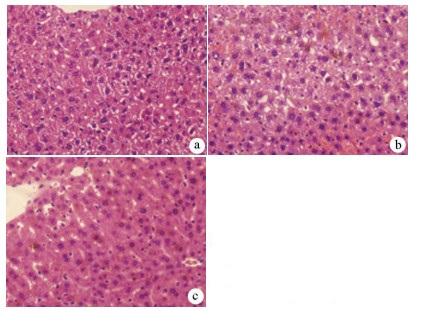
目的 探讨丹参酮Ⅰ(T-Ⅰ)在小鼠肝缺血再灌注损伤(HIRI)模型中的保护作用。 方法 C57BL/6J小鼠36只随机分为假手术(sham)组(n=6)、缺血再灌注(IR)组(n=6)、IR+T-Ⅰ(5 mg/kg)组(n=6)、IR+T-Ⅰ(10 mg/kg)组(n=6)、IR+T-Ⅰ(20 mg/kg)组(n=6)和IR+T-Ⅰ(40 mg/kg)组(n=6),各组均腹腔注射给药,sham组与IR组注射等量溶剂橄榄油,IR+T-Ⅰ组每日给药1次,连续给药7 d, 末次给药2 h后建立70%的HIRI模型,再灌注6 h后收集血清及肝脏标本; 试剂盒检测血清ALT、AST水平,检测肝组织内超氧化物歧化酶(SOD)、丙二醛(MDA)、Caspase-3及还原型谷胱甘肽(GSH)指标; HE染色观察肝组织病理情况,TUNEL法检测肝细胞凋亡水平,免疫组化检测Caspase-3、血红素加氧酶-1(HO-1)蛋白表达水平。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 IR+T-Ⅰ(20 mg/kg)组的血清ALT[(192.48±23.67)U/L]、AST[(123.19±9.16)U/L]较IR组[ALT:(336.90±41.52)U/L, AST:(206.90±18.81)U/L]均显著下降(P值均<0.01),确定了20 mg/kg为最佳浓度; 与IR组[MDA:(3.48±0.95)μmol/mg; Caspase-3:(1.04±0.35)μmol/mg; SOD:(160.29±27.37)U/mg; GSH:(1.03±0.42)μmol/mg]比较,IR+T-Ⅰ(20 mg/kg)组的MDA[(1.34±0.21)μmol/mg]、Caspase-3[(0.69±0.97)μmol/mg]均显著降低(P值均<0.05),而SOD[(274.47±30.53)U/mg]及GSH[(2.12±0.27)μmol/mg]均明显升高(P值均<0.05);HE染色显示,IR组肝小叶结构紊乱,肝细胞灶性或大面积变性坏死; 与IR组相比,IR+T-Ⅰ(20 mg/kg)组肝细胞坏死面积减小,肝组织结构基本完整; 免疫组化结果显示,与IR组比较,IR+T-Ⅰ(20 mg/kg)组的小鼠肝细胞凋亡数目明显减少,Caspase-3蛋白表达明显减少,HO-1蛋白表达明显增加。 结论 T-Ⅰ通过抑制肝脏氧化应激反应和肝细胞凋亡对小鼠HIRI中起到保护作用。 Abstract:Objective To investigate the protective effect of tanshinone I (T-I) on hepatic ischemia-reperfusion injury (HIRI) in mice. Methods A total of 36 C57BL/6J mice were randomly divided into sham-operation group, ischemia-reperfusion (IR) group, IR+T-I (5 mg/kg) group, IR+T-I (10 mg/kg) group, IR+T-I (20 mg/kg) group, and IR+T-I (40 mg/kg) group, with 6 mice in each group. Each group was given intraperitoneal injection. The mice in the sham-operation group and the IR group were injected with an equal volume of the solvent olive oil; the mice in the IR+T-I groups were administered once a day for 7 consecutive days, a model of 70% HIRI was established at 2 hours after the last administration, and serum and liver samples were collected after 6 hours of reperfusion. Related kits were used to measure the serum level of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and the content of superoxide dismutase (SOD), malondialdehyde (MDA), caspase-3, and reduced glutathione (GSH) in liver tissue; HE staining was used to observe liver histopathology; the TUNEL method was used to measure the level of hepatocyte apoptosis; immunohistochemistry was used to measure the protein expression of caspase-3 and heme oxygenase-1 (HO-1). A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results Compared with the IR group, the IR+T-I (20mg/kg) group had significant reductions in the serum levels of ALT (192.48±23.67 U/L vs 336.90±41.52 U/L, P < 0.01) and AST (123.19±9.16 U/L vs 206.90±18.81 U/L, P < 0.01), and thus 20 mg/kg was determined as the optimal concentration. Compared with the IR group, the IR+T-I (20 mg/kg) group had significant reductions in MDA (1.34±0.21 μmol/mg vs 3.48±0.95 μmol/mg, P < 0.05) and caspase-3 (0.69±0.97 μmol/mg vs 1.04±0.35 μmol/mg, P < 0.05) and significant increases in SOD (274.47±30.53 U/mg vs 160.29±27.37 U/mg, P < 0.05) and GSH (2.12±0.27 μmol/mg vs 1.03±0.42 μmol/mg, P < 0.05). HE staining showed that the IR group had disordered structure of hepatic lobules and focal or extensive degeneration and necrosis of hepatocytes; compared with the IR group, the IR+T-I (20 mg/kg) group had a reduction in the area of hepatocyte necrosis and a basically complete structure of the liver. Immunohistochemistry showed that compared with the IR group, the IR+T-I (20 mg/kg) group had significant reductions in the number of apoptotic hepatocytes and the protein expression of caspase-3 and a significant increase in the protein expression of HO-1. Conclusion T-I exerts a protective effect against HIRI in mice by inhibiting liver oxidative stress response and hepatocyte apoptosis. -
Key words:
- Reperfusion Injury /
- Oxidative Stress /
- TANSHINONE
-
表 1 5组间ALT、AST水平的比较
指标 Sham组
(n=6)IR组
(n=6)IR+T-Ⅰ
(5 mg/kg)
(n=6)IR+T-Ⅰ
(10 mg/kg)
(n=6)IR+T-Ⅰ
(20 mg/kg)
(n=6)IR+T-Ⅰ
(40 mg/kg)
(n=6)F值 P值 ALT(U/L) 18.64±3.72 336.90±41.521) 287.09±18.47 208.83±29.112) 192.48±23.672) 220.47±19.802) 87.06 <0.01 AST(U/L) 34.59±12.71 206.90±18.811) 174.25±16.072) 145.78±8.392) 123.19±9.162) 137.09±22.072) 69.14 <0.01 注:1)与Sham组比较,P<0.01; 2)与IR组比较,P<0.05。 表 2 3组间MDA、SOD、GSH、Caspase-3水平的比较
指标 Sham组
(n=6)IR组
(n=6)IR+T-Ⅰ(20 mg/kg)组
(n=6)F值 P值 SOD(U/mg) 345.08±43.81 160.29±27.371) 274.47±30.532) 27.76 <0.01 MDA(μmol/mg) 0.86±0.17 3.48±0.951) 1.34±0.212) 31.15 <0.01 GSH(μmol/mg) 2.76±0.09 1.03±0.421) 2.12±0.272) 36.62 <0.01 Caspase-3(μmol/mg) 0.47±0.43 1.04±0.351) 0.69±0.972) 85.99 <0.01 注:1)与Sham组比较,P<0.01; 2)与IR组比较,P<0.05。 -
[1] YANG L, WANG W, WANG X, et al. Creg in hepatocytes ameliorates liver ischemia/reper fusion injury in a TAK1-dependent manner in mice[J]. Hepatology, 2019, 69(1): 294-313. DOI: 10.1002/hep.30203 [2] ZHANG S, JIANG S, WANG H, et al. SIRT6 protects against hepatic ischemia/reperfusion injury by inhibiting apoptosis and autophagy related cell death[J]. Free Radic Biol Med, 2018, 115: 18-30. DOI: 10.1016/j.freeradbiomed.2017.11.005 [3] ZAKI AM, EL-TANBOULY DM, ABDELSALAM RM, et al. Plumbagin ameliorates hepatic ischemia-reperfusion injury in rats: Role of high mobility group box 1 in inflammation, oxidative stress and apoptosis[J]. Biomed Pharmacother, 2018, 106: 785-793. DOI: 10.1016/j.biopha.2018.07.004 [4] KATWAL G, BARAL D, FAN X, et al. SIRT3 a major player in attenuation of hepatic ischemia-reperfusion injury by reducing ROS via its downstream mediators: SOD2, CYP-D, and HIF-1 α[J]. Oxid Med Cell Longev, 2018, 2018: 2976957. http://downloads.hindawi.com/journals/omcl/2018/2976957.pdf [5] HAN JY, FAN JY, HORIE Y, et al. Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion[J]. Pharmacol Ther, 2008, 117(2): 280-295. DOI: 10.1016/j.pharmthera.2007.09.008 [6] ZHOU L, ZUO Z, CHOW MS. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use[J]. J Clin Pharmacol, 2005, 45(12): 1345-1359. DOI: 10.1177/0091270005282630 [7] PARK JH, OK P, CHO JH, et al. Anti-inflammatory effect of tanshinone I in neuroprotection against cerebral ischemia-reperfusion injury in the gerbil hippocampus[J]. Neurochem Res, 2014, 39(7): 1300-1312. DOI: 10.1007/s11064-014-1312-4 [8] WANG S, JING H, YANG H, et al. Tanshinone I selectively suppresses pro-inflammatory genes expression in activated microglia and prevents nigrostriatal dopaminergic neurodegeneration in a mouse model of Parkinson's disease[J]. J Ethnopharmacol, 2015, 164: 247-255. DOI: 10.1016/j.jep.2015.01.042 [9] JING X, WEI X, REN M, et al. Neuroprotective effects of Tanshinone I against 6-OHDA- induced oxidative stress in cellular and mouse model of parkinson's disease through upregulating Nrf2[J]. Neurochem Res, 2016, 41(4): 779-786. DOI: 10.1007/s11064-015-1751-6 [10] TAO S, ZHENG Y, LAU A, et al. Tanshinone I activates the Nrf2-dependent antioxidant response and protects against As(Ⅲ)-induced lung inflammation in vitro and in vivo[J]. Antioxid Redox Signal, 2013, 19(14): 1647-1661. DOI: 10.1089/ars.2012.5117 [11] GAO L, QIAN BL, CHEN H, et al. Hic-5 deficiency attenuates hepatic ischemia reperfusion injury through TLR4/NF-κB signaling pathways[J]. Life Sci, 2020, 249: 117517. DOI: 10.1016/j.lfs.2020.117517 [12] KONISHI T, LENTSCH AB. Hepatic ischemia/reperfusion: Mechanisms of tissue injury, repair, and regeneration[J]. Gene Expr, 2017, 17(4): 277-287. DOI: 10.3727/105221617X15042750874156 [13] LI J, LI RJ, LV GY, et al. The mechanisms and strategies to protect from hepatic ischemia-reperfusion injury[J]. Eur Rev Med Pharmacol Sci, 2015, 19(11): 2036-2047. http://www.ncbi.nlm.nih.gov/pubmed/26125267 [14] LIANG R, NICKKHOLGH A, KERN M, et al. Green tea extract ameliorates reperfusion injury to rat livers after warm ischemia in a dose-dependent manner[J]. Mol Nutr Food Res, 2011, 55(6): 855-863. DOI: 10.1002/mnfr.201000643 [15] HASSAN-KHABBAR S, COTTART CH, WENDUM D, et al. Postischemic treatment by trans-resveratrol in rat liver ischemia-reperfusion: A possible strategy in liver surgery[J]. Liver Transpl, 2008, 14(4): 451-459. DOI: 10.1002/lt.21405 [16] CHENG F, LI Y, FENG L, et al. Effects of tetrandrine on ischemia/reperfusion injury in mouse liver[J]. Transplant Proc, 2008, 40(7): 2163-2166. DOI: 10.1016/j.transproceed.2008.07.082 [17] GAO WQ, QIU XF, LI K, et al. Protective effect of Tanshinone I against renal ischemia /reperfusion injury[J]. J Southeast Univ(Med Sci Edi), 2018, 37(3): 372-379. (in Chinese) DOI: 10.3969/j.issn.1671-6264.2018.03.002高文强, 邱雪峰, 李凯, 等. 丹参酮Ⅰ在肾脏缺血再灌注损伤中的保护作用研究[J]. 东南大学学报(医学版), 2018, 37(3): 372-379. DOI: 10.3969/j.issn.1671-6264.2018.03.002 [18] LI X, WU Y, ZHANG W, et al. Pre-conditioning with tanshinone ⅡA attenuates the ischemia/reperfusion injury caused by liver grafts via regulation of HMGB1 in rat Kupffer cells[J]. Biomed Pharmacother, 2017, 89: 1392-1400. DOI: 10.1016/j.biopha.2017.03.022 [19] QI YY, XIAO L, ZHANG LD, et al. Tanshinone ⅡA pretreatment attenuates hepatic ischemia-reperfusion[J]. Front Biosci (Elite Ed), 2012, 4: 1303-1313. [20] WANG QQ, ZHAO X, CHEN YC, et al. Research advances in mechanisms and intervention of hepatic ischemia-reperfusion injury[J]. J Clin Hepatol, 2016, 32(6): 1225-1229. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2016.06.049王清卿, 赵鑫, 陈玉超, 等. 肝脏缺血再灌注损伤机制及干预的研究进展[J]. 临床肝胆病杂志, 2016, 32(6): 1225-1229. DOI: 10.3969/j.issn.1001-5256.2016.06.049 [21] WANG JY, LI SW, GONG JP, et al. Role of silent information regulator 1 in hepatic ischemia-reperfusion injury[J]. J Clin Hepatol, 2019, 35(6): 1388-1391. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2019.06.047王敬元, 李生伟, 龚建平, 等. 沉默信息调节因子1在肝脏缺血再灌注损伤中的作用[J]. 临床肝胆病杂志, 2019, 35(6):1388-1391. DOI: 10.3969/j.issn.1001-5256.2019.06.047 [22] ZHANG H, FORMAN HJ. Glutathione synthesis and its role in redox signaling[J]. Semin Cell Dev Biol, 2012, 23(7): 722-728. DOI: 10.1016/j.semcdb.2012.03.017 [23] LU SC. Glutathione synthesis[J]. Biochim Biophys Acta, 2013, 1830(5): 3143-3153. DOI: 10.1016/j.bbagen.2012.09.008 [24] HUANG TT, ZOU Y, CORNIOLA R. Oxidative stress and adult neurogenesis-effects of radiation and superoxide dismutase deficiency[J]. Semin Cell Dev Biol, 2012, 23(7): 738-744. DOI: 10.1016/j.semcdb.2012.04.003 [25] SIEMS W, QUAST S, CARLUCCIO F, et al. Oxidative stress in chronic renal failure as a cardiovascular risk factor[J]. Clin Nephrol, 2002, 58(Suppl 1): s12-s19. http://europepmc.org/abstract/MED/12227720 [26] ATEF Y, EL-FAYOUMI HM, ABDEL-MOTTALEB Y, et al. Quercetin and tin protoporphyrin attenuate hepatic ischemia reperfusion injury: Role of HO-1[J]. Naunyn Schmiedebergs Arch Pharmacol, 2017, 390(9): 871-881. DOI: 10.1007/s00210-017-1389-9 [27] CHENG Y, RONG J. Therapeutic potential of heme oxygenase-1/carbon monoxide system against ischemia-reperfusion injury[J]. Curr Pharm Des, 2017, 23(26): 3884-3898. http://www.ncbi.nlm.nih.gov/pubmed/28412905 [28] LIU X, LIU J. Tanshinone I induces cell apoptosis by reactive oxygen species-mediated endoplasmic reticulum stress and by suppressing p53/DRAM-mediated autophagy in human hepatocellular carcinoma[J]. Artif Cells Nanomed Biotechnol, 2020, 48(1): 488-497. DOI: 10.1080/21691401.2019.1709862 [29] ESTOLANO-COBIÁN A, ALONSO MM, DÍAZ-RUBIO L, et al. Tanshinones and their derivatives: Heterocyclic ring-fused diterpene of biological interest[J]. Mini Rev Med Chem, 2020.[Online ahead of print] -



 PDF下载 ( 2500 KB)
PDF下载 ( 2500 KB)

 下载:
下载: