肝爽颗粒浸膏减轻对乙酰氨基酚所致肝细胞损伤的作用机制
DOI: 10.3969/j.issn.1001-5256.2021.01.024
Mechanism of Ganshuang granule extract in alleviating N-acetyl-p-aminophenol-induced hepatocellular injury
-
摘要:
目的 研究肝爽颗粒浸膏减轻对乙酰氨基酚(N-乙酰基-对氨基苯酚,APAP)诱导的肝细胞毒性的能力,以及可能涉及的机制。 方法 设立5组细胞培养组,分别为正常对照组、APAP损伤组、3组不同肝爽颗粒浸膏浓度的损伤保护组。使用20 mmol/L APAP加入细胞培养液孵育24 h构建造体外药物肝损伤模型,损伤保护组提前加用不同浓度肝爽颗粒浸膏(0.2 μg/ml、1 μg/ml、5 μg/ml)8 h孵育后加入APAP损伤24 h。检测不同组别的肝细胞损伤标志物(ALT、AST、LDH)、线粒体损伤标志物(线粒体膜电位、GDH)、抗氧化及氧化应激标志物(GSH、SOD、MDA、ROS)等。进一步针对实验结果进行机制探讨。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 肝爽颗粒浸膏可以减轻APAP引起的肝细胞毒性,肝爽颗粒浸膏可以提高细胞活力(P<0.001),降低上清中AST、ALT、LDH的含量(P值分别为<0.001、<0.001、<0.05);肝爽颗粒浸膏可以抑制APAP诱导的肝细胞氧化应激,肝爽颗粒浸膏组的氧化应激指标ROS、MDA较APAP组下降(P值均<0.01);肝爽颗粒浸膏可以剂量依赖性减轻APAP诱导的肝细胞线粒体膜电位丢失(P<0.05),降低了上清线粒体损伤标志物GDH的含量(P<0.001);肝爽颗粒浸膏可以抑制CYP2E1/1A2的表达(P值均<0.05);肝爽颗粒浸膏可以增加肝细胞Ⅱ相酶表达; 肝爽颗粒浸膏可以诱导Nrf2及其下游基因NQO-1及GCLC的表达(P值均<0.05)。 结论 肝爽颗粒浸膏可通过两种途径预防APAP诱导肝脏损伤,第一种途径是肝爽颗粒浸膏下调CYP2E1/1A2的表达减少了APAP毒性产物NAPQI的生成; 第二种途径是肝爽颗粒浸膏上调解毒通路的表达,激活Nrf2增加抗氧化酶(SOD、GSH)和Ⅱ相酶的表达,从而加速APAP的无害代谢。 Abstract:Objective To investigate the ability of Ganshuang granule (a liver-protecting drug widely used in clinical practice) extract to reduce N-acetyl-p-aminophenol (APAP)-induced hepatotoxicity and possible mechanisms. Methods A total of five cell culture groups were set up in this experiment, i.e., normal control group, APAP injury group, and three injury protection groups treated with different concentrations of Ganshuang granule extract. Then 20 mmol/L APAP was added to the cell culture medium and incubated for 24 hours to establish an in vitro model of drug-induced liver injury, and the injury protection groups were treated with different concentrations of Ganshuang granule extract (0.2, 1, and 5 μg/ml) in advance for 8 hours of incubation before APAP were added for 24 hours. Related markers were measured, including the markers for hepatocellular injury [alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH)], the markers for mitochondrial injury [mitochondrial membrane potential, and glutamate dehydrogenase (GDH)], and antioxidant and oxidative stress markers [glutathione (GSH), superoxide dismutase (SOD), malondialdehyde (MDA), and reactive oxygen species (ROS)]. Related mechanism was discussed based on the experimental results. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results Ganshuang granule extract alleviated APAP-induced hepatotoxicity, improved cell viability (P < 0.001), and reduced the levels of AST, ALT, and LDH in supernatant (P < 0.001, P < 0.001, and P < 0.05). Ganshuang granule extract inhibited APAP-induced hepatocellular oxidative stress, and compared with the APAP group, the Ganshuang granule extract groups had significant reductions in the oxidative stress indicators ROS and MDA (both P < 0.01). Ganshuang granule extract alleviated the loss of mitochondrial membrane potential induced by APAP (P < 0.05) and reduced the content of the mitochondrial injury marker GDH in supernatant (P < 0.001) in a dose-dependent manner. Ganshuang granule extract inhibited the expression of CYP2E1/1A2 (both P < 0.05) and increased the expression of phase Ⅱ enzymes in hepatocytes. Ganshuang granule extract induced the expression of Nrf2 and its downstream genes NQO-1 and GCLC (all P < 0.05). Conclusion Ganshuang granule extract can prevent APAP-induced hepatocellular injury through two ways. The first way is that Ganshuang granule extract downregulates the expression of CYP2E1/1A2 and thus reduces the production of NAPQI, a toxic product of APAP; the second way is that Ganshuang granule extract upregulates the expression of the detoxification pathway, which can activate Nrf2 to increase the expression of antioxidant enzymes (SOD and GSH) and phase Ⅱ enzymes and thus accelerate the harmless metabolism of APAP. -
Key words:
- Drug-Induced Liver Injury /
- Acetaminophen /
- Ganshuang Granules
-
表 1 引物序列
引物名称 序列(5′-3′) GAPDH F GGACTCATGACCACAGTCCA R TCAGCTCAGGGATGACCTTG UGT1A1 F CGCCTCTCCAGCCTTCACAAG R GTCAGCACGACGGCCAAGAG UGT1A3 F GCCAACAGGAAGCCACTATC R CAGCAATTGCCATAGCTTTC UGT1A6 F CCAGTGCCGTATGACCAAGAAGAG R GGAGGCTCTGGCAGTTGATGAAG UGT2B7 F GCAGCAGAATACAGCCATTGGATG R GCTGAAGATGCCAGTACAGTCACC GSTα1 F TCCAGCTTCCCTCTGCTGAA R GGCTGCCAGGCTGTAGAAAC GSTM1 F GCCTGCTCCTGGAATACACAGAC R GCAATGTAGCACAAGATGGCGTTG SULT1A1 F TGGTTCAGCACACGTCGTTCAAG R CATCTTCTCCGCATAGTCCGCATC SULT2A1 F GAGATTCTCTGCCTGATGCACTCC R ATCACCTTGGCCTTGGAACTGAAG GCLC F ATGGCTTTGAGTGCTGCATCTCC R GGCTCCAGTCCTCGCTCCTC NQO1 F GTCGGCAGAAGAGCACTGATCG R ACTCCACCACCTCCCATCCTTTC Nrf2 F ACACGGTCCACAGCTCATCAT R TTGGCTTCTGGACTTGGAAC CYP2E1 F GCCGACATCCTCTTCCGCAAG R CTGTGGCTTCCAGGCAAGTAGTG CYP1A2 F CAACACCTTCTCCATCGCCTCTG R CCACTGACACCACCACCTGATTG 表 2 肝爽浸膏对APAP诱导的HepG2细胞急性损伤的影响
指标 对照组 APAP组 浸膏0.2 μg/ml组 浸膏1 μg/ml组 浸膏5 μg/ml组 F值 P值 细胞活力(%) 100.00±3.33 37.53±0.51 40.45±0.58 45.02±0.07 43.69±0.80 1668.01 <0.001 AST(U/ml) 3.80±0.14 7.80±0.14 7.50±0.42 6.70±0.14 6.90±0.14 97.80 <0.001 ALT(U/ml) 0.55±0.07 1.55±0.07 1.10±0.14 0.85±0.07 0.70±0.00 43.92 <0.001 LDH(U/ml) 34.25±5.44 56.70±3.25 38.30±5.66 42.35±1.34 35.90±3.25 9.63 <0.05 表 3 肝爽浸膏对APAP诱导的氧化应激指标的影响
指标 对照组 APAP组 浸膏0.2 μg/ml组 浸膏1 μg/ml组 浸膏5 μg/ml组 F值 P值 GSH(μmol/g prot) 51.79±9.06 37.14±1.43 47.14±6.23 49.29±4.88 43.21±3.76 3.289 <0.05 SOD(U/mg prot) 319.74±5.58 245.00±1.77 284.63±0.87 281.25±11.46 281.78±14.68 13.454 <0.05 ROS(%) 100.00±1.94 277.94±1.41 262.19±1.92 238.47±3.15 231.92±2.80 919.150 <0.001 MDA(nmol/mg prot) 0.84±0.12 3.70±0.48 1.00±0.12 1.81±0.21 1.08±0.12 66.900 <0.001 表 4 肝爽浸膏对APAP诱导的线粒体损伤的影响
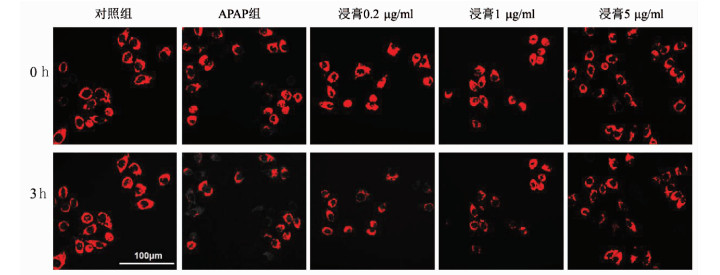
指标 对照组 APAP组 浸膏0.2 μg/ml组 浸膏1 μg/ml组 浸膏5 μg/ml组 F值 P值 荧光表达 1.38±0.04 0.28±0.03 0.34±0.03 0.59±0.08 0.76±0.11 35.868 <0.05 GDH(IU/ml) 43.51±0.37 60.66±1.96 56.26±0.45 54.98±0.45 53.37±0.91 89.590 <0.001 表 5 肝爽浸膏对不同组别CYP2E1/1A2 mRNA表达水平的影响
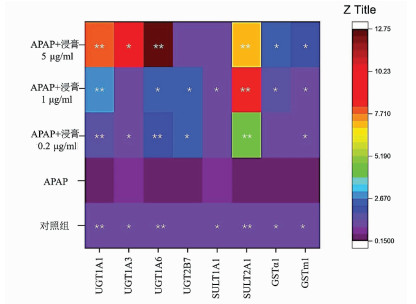
指标 对照组 APAP组 浸膏0.2 μg/ml组 浸膏1 μg/ml组 浸膏5 μg/ml组 F值 P值 CYP2E1 1.00±0.05 1.89±0.31 0.86±0.07 0.64±0.06 0.70±0.09 32.909 <0.001 CYP1A2 1.00±0.01 1.13±0.11 0.95±0.09 0.67±0.02 0.58±0.05 22.710 <0.05 表 6 肝爽浸膏对Nrf2、GCLC及NQO-1 mRNA水平的影响
指标 对照组 APAP组 浸膏0.2 μg/ml组 浸膏1 μg/ml组 浸膏5 μg/ml组 F值 P值 Nrf2 1.00±0.04 0.13±0.01 0.61±0.04 0.75±0.00 0.76±0.15 39.084 <0.05 NQO-1 1.00±0.08 0.29±0.02 0.46±0.05 0.78±0.06 0.82±0.15 22.456 <0.05 GCLC 1.00±0.01 0.51±0.09 1.32±0.25 1.29±0.05 1.19±0.18 10.464 <0.05 -
[1] HOLUBEK WJ, KALMAN S, HOFFMAN RS. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study[J]. Hepatology, 2006, 43(4): 880; author reply 882. [2] ZHANG XW, ZHENG SQ, MA YZ, et al. Effects of artichoke on acetaminophen-induced drug-induced liver injury in rats[J]. Chin J Immunol, 2020, 36(17): 2096-2099.(in Chinese) DOI: 10.3969/j.issn.1000-484X.2020.17.010张晓文, 郑思琪, 马应卓, 等. 洋蓟素对对乙酰氨基酚诱导的大鼠药物性肝损伤的影响[J]. 中国免疫学杂志, 2020, 36(17): 2096-2099. DOI: 10.3969/j.issn.1000-484X.2020.17.010 [3] BLIEDEN M, PARAMORE LC, SHAH D, et al. A perspective on the epidemiology of acetaminophen exposure and toxicity in the United States[J]. Expert Rev Clin Pharmacol, 2014, 7(3): 341-348. DOI: 10.1586/17512433.2014.904744 [4] ZHOU Y, YANG L, LIAO Z, et al. Epidemiology of drug-induced liver injury in China: A systematic analysis of the Chinese literature including 21, 789 patients[J]. Eur J Gastroenterol Hepatol, 2013, 25(7): 825-829. DOI: 10.1097/MEG.0b013e32835f6889 [5] HARDWICK RN, FERREIRA DW, MORE VR, et al. Altered UDP-glucuronosyltransferase and sulfotransferase expression and function during progressive stages of human nonalcoholic fatty liver disease[J]. Drug Metab Dispos, 2013, 41(3): 554-561. DOI: 10.1124/dmd.112.048439 [6] YU PF, WU Q, DUAN ZP, et al. Research advances in the mechanism of drug-induced liver injury due to paracetamol[J]. J Clin Hepatol, 2019, 35(9): 2108-2112. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2019.09.050余朋飞, 吴桥, 段钟平, 等. 对乙酰氨基酚致药物性肝损伤的机制研究进展[J]. 临床肝胆病杂志, 2019, 35(9): 2108-2112. DOI: 10.3969/j.issn.1001-5256.2019.09.050 [7] SUN HQ, WANG XQ, SHI HB, et al. Ganshuang granules protect mouse liver from chronic injury induced by CCl4 via autophagy[J]. J Clin Hepatol, 2015, 31(7): 130-135. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2015.07.028孙海青, 王小琪, 时红波, 等. 肝爽颗粒对CCl4诱导的慢性肝损伤小鼠模型和肝损伤细胞模型的保护作用[J]. 临床肝胆病杂志, 2015, 31(7): 130-135. DOI: 10.3969/j.issn.1001-5256.2015.07.028 [8] KANG WW, ZHOU L, DANG SS, et al. Multi center clinical study on the treatment of chronic hepatitis B with Ganshuang Granules[J]. China J Tradit Chin Med Pharma, 2017, 32(12): 5689-5693. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-BXYY201712121.htm康玮玮, 周莉, 党双锁, 等. 肝爽颗粒治疗慢性乙型肝炎的多中心临床研究[J]. 中华中医药杂志, 2017, 32(12): 5689-5693. https://www.cnki.com.cn/Article/CJFDTOTAL-BXYY201712121.htm [9] KANG SW, HAYDAR G, TANIANE C, et al. AMPK activation prevents and reverses drug-induced mitochondrial and hepatocyte injury by promoting mitochondrial fusion and function[J]. PLoS One, 2016, 11(10): e0165638. DOI: 10.1371/journal.pone.0165638 [10] SHI H, SHI H, REN F, et al. Naringin in Ganshuang Granule suppresses activation of hepatic stellate cells for anti-fibrosis effect by inhibition of mammalian target of rapamycin[J]. J Cell Mol Med, 2017, 21(3): 500-509. DOI: 10.1111/jcmm.12994 [11] HERNÁNDEZ-AQUINO E, MURIEL P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms[J]. World J Gastroenterol, 2018, 24(16): 1679-1707. DOI: 10.3748/wjg.v24.i16.1679 [12] HINSON JA, MICHAEL SL, AULT SG, et al. Western blot analysis for nitrotyrosine protein adducts in livers of saline-treated and acetaminophen-treated mice[J]. Toxicol Sci, 2000, 53(2): 467-473. DOI: 10.1093/toxsci/53.2.467 [13] ZHAO H, JIANG Z, CHANG X, et al. 4-Hydroxyphenylacetic acid prevents acute APAP-induced liver injury by increasing phase Ⅱ and antioxidant enzymes in mice[J]. Front Pharmacol, 2018, 9: 653. DOI: 10.3389/fphar.2018.00653 [14] FAN X, LV H, WANG L, et al. Isoorientin Ameliorates APAP-induced hepatotoxicity via activation Nrf2 antioxidative pathway: The involvement of AMPK/Akt/GSK3β[J]. Front Pharmacol, 2018, 9: 1334. DOI: 10.3389/fphar.2018.01334 [15] FAKURAZI S, SHARIFUDIN SA, ARULSELVAN P. Moringa oleifera hydroethanolic extracts effectively alleviate acetaminophen-induced hepatotoxicity in experimental rats through their antioxidant nature[J]. Molecules, 2012, 17(7): 8334-8350. DOI: 10.3390/molecules17078334 [16] OKAWA H, MOTOHASHI H, KOBAYASHI A, et al. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity[J]. Biochem Biophys Res Commun, 2006, 339(1): 79-88. DOI: 10.1016/j.bbrc.2005.10.185 [17] KLAASSEN CD, REISMAN SA. Nrf2 the rescue: Effects of the antioxidative/electrophilic response on the liver[J]. Toxicol Appl Pharmacol, 2010, 244(1): 57-65. DOI: 10.1016/j.taap.2010.01.013 [18] GUM SI, CHO MK. Recent updates on acetaminophen hepatotoxicity: The role of nrf2 in hepatoprotection[J]. Toxicol Res, 2013, 29(3): 165-172. DOI: 10.5487/TR.2013.29.3.165 [19] LIN M, ZHAI X, WANG G, et al. Salvianolic acid B protects against acetaminophen hepatotoxicity by inducing Nrf2 and phase Ⅱ detoxification gene expression via activation of the PI3K and PKC signaling pathways[J]. J Pharmacol Sci, 2015, 127(2): 203-210. DOI: 10.1016/j.jphs.2014.12.010 [20] GOKILA VANI M, KUMAR KJ, LIAO JW, et al. Antcin C from antrodia cinnamomea protects liver cells against free radical-induced oxidative stress and apoptosis in vitro and in vivo through Nrf2-dependent mechanism[J]. Evid Based Complement Alternat Med, 2013, 2013: 296082. 期刊类型引用(3)
1. 张艳培,梁健,邓鑫,梁明坤,张冰冰,徐粤娟. 浅析肝纤维化发生机制. 大众科技. 2021(02): 41-44+12 .  百度学术
百度学术2. 曾静,熊庆,张丽丽,袁娟,何莹,邱黎菲. SPACE肽修饰成纤维细胞生长因子21的制备及对小鼠皮肤瘢痕形成的抑制作用. 中国免疫学杂志. 2021(11): 1308-1312 .  百度学术
百度学术3. 唐青蓝,李红梅,张礼林,王玉丰,颜礼完,周君. 小鼠FGF21基因克隆及his-mFGF21融合蛋白的诱导表达. 贵州医科大学学报. 2019(05): 552-556 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 3021 KB)
PDF下载 ( 3021 KB)

 下载:
下载:

 百度学术
百度学术

