茵陈蒿汤调控lncRNA PVT1/miRNA-30a-5p信号通路对重症急性胰腺炎大鼠模型的保护作用
DOI: 10.3969/j.issn.1001-5256.2021.01.029
Artemisia capillaris Thunb. decoction exerts a protective effect on rats with severe acute pancreatitis by regulating the lncRNA PVT1/miRNA-30a-5p signaling pathway
-
摘要:
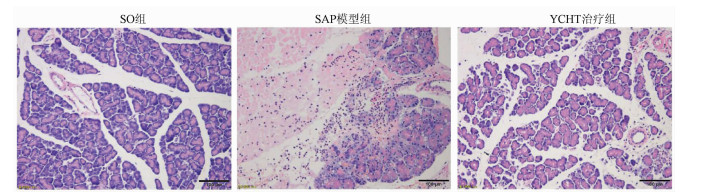
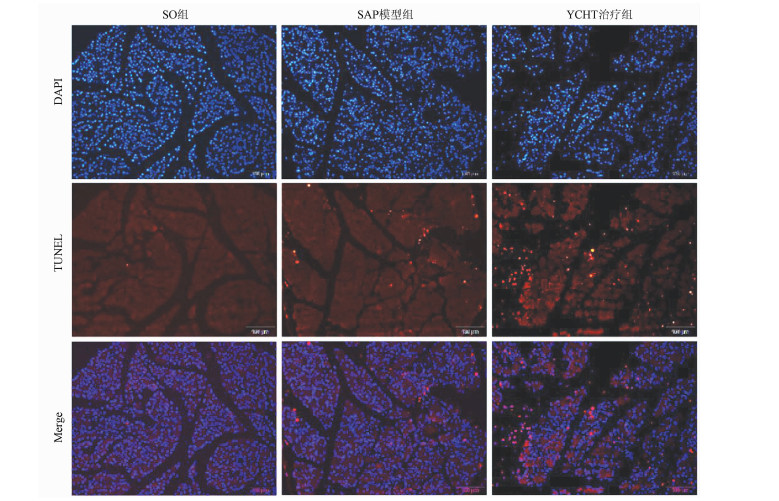
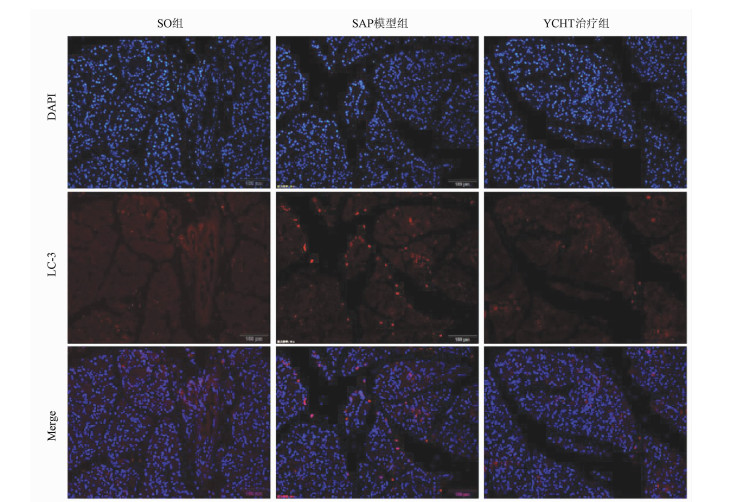
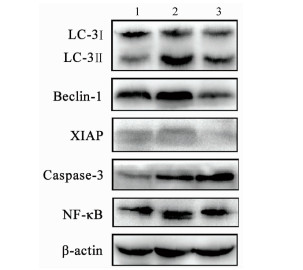
目的 探讨清热利胆的经典中药方剂——茵陈蒿汤(YCHT)对牛磺胆酸钠诱导的大鼠重症急性胰腺炎(SAP)的保护作用及其机制。 方法 30只SD大鼠随机分成假手术(SO)组、SAP模型组和YCHT (4.0 g/kg)治疗组,每组10只。造模成功24 h后,留取大鼠胰腺组织和血浆待检测。HE染色观察胰腺病理损伤情况; ELISA法检测血浆淀粉酶、TNFα和IL-1β水平; 免疫荧光染色检测LC-3蛋白的荧光强度,TUNEL检测细胞凋亡情况。Western Blot检测胰腺组织LC-3、Beclin-1、X连锁凋亡抑制蛋白(XIAP)、Caspase-3和NF-κB蛋白表达。实时荧光定量PCR检测lncRNA PVT1和miRNA-30a-5p的表达水平。采用单因素方差分析和Tukey’s检验用于分析多个独立样本之间的差异。 结果 YCHT能明显减轻SAP大鼠胰腺组织水肿、坏死、出血及炎性细胞浸润等病理学损伤。与SO组相比,SAP模型组大鼠血浆淀粉酶、炎性因子TNFα和IL-1β水平显著升高,YCHT治疗后血浆淀粉酶及TNFα和IL-1β水平显著降低(P值均<0.05)。与SO组相比,SAP模型组LC-3Ⅱ/LC-3Ⅰ比值及Beclin-1、XIAP、Caspase-3和NF-κB蛋白表达明显上调,YCHT治疗组LC-3Ⅱ/LC-3Ⅰ、Beclin-1、XIAP和NF-κB的表达水平较SAP模型组显著降低,Caspase-3水平显著升高(P值均<0.05)。与SO组相比,SAP模型组大鼠胰腺lncRNA PVT1表达显著升高,miRNA-30a-5p表达显著降低(P值均<0.05);与SAP模型组相比,YCHT显著降低了lncRNA PVT1的表达,增加了miRNA-30a-5p的表达(P值均<0.05)。 结论 lncRNA PVT1/miRNA-30a-5p介导的细胞自噬和凋亡可能是YCHT治疗SAP的一个药物靶点,这为进一步开发中药方剂YCHT治疗SAP提供了实验基础和理论依据。 -
关键词:
- 胰腺炎 /
- 大鼠,Sprague-Dawley /
- 茵陈蒿汤 /
- RNA,长链非编码 /
- 微RNAs
Abstract:Objective To investigate the protective effect and mechanism of Artemisia capillaris Thunb. decoction (YCHT), a classic heat-clearing and cholagogic traditional Chinese medicine (TCM) prescription, on rats with severe acute pancreatitis (SAP) induced by sodium taurocholate. Methods A total of 30 Sprague-Dawley rats were randomly divided into sham-operation (SO) group, SAP model group, and YCHT (4.0 g/kg) treatment group, with 10 rats in each group. At 24 hours after successful modeling, pancreatic tissue and plasma samples were collected for analysis. HE staining was used to observe pathological injury of the pancreas; ELISA was used to measure the plasma levels of amylase, tumor necrosis factor-α (TNFα), and interleukin-1β (IL-1β); immunofluorescent staining was used to measure the fluorescence intensity of LC-3 protein, and TUNEL was used to measure cell apoptosis. Western blot was used to measure the protein expression of LC-3, Beclin-1, X-linked inhibitor of apoptosis protein (XIAP), caspase-3, and nuclear factor-kappa B (NF-κB) in the pancreas, and quantitative real-time PCR was used to measure the expression levels of lncRNA PVT1 and miRNA-30a-5p. A one-way analysis of variance and the Tukey's test were used to analyze the differences between multiple independent samples. Results YCHT significantly alleviated the pathological injury of the pancreas of SAP rats, such as edema, necrosis, hemorrhage, and inflammatory cell infiltration. Compared with the SO group, the SAP group had significant increases in the plasma levels of amylase and the inflammatory factors TNFα and IL-1β, and there were significant reductions in the plasma levels of amylase, TNFα, and IL-1β after YCHT treatment (all P < 0.05). Compared with the SO group, the SAP group had significant increases in LC-3II/LC-3I ratio and the protein expression of Beclin-1, XIAP, caspase-3, and NF-κB, and compared with the SAP group, the YCHT group had significant reductions in LC-3II/LC-3I ratio and the protein expression of Beclin-1, XIAP, and NF-κB (all P < 0.05). Compared with the SO group, the SAP group had a significant increase in the expression of lncRNA PVT1 and a significant reduction in the expression of miRNA-30a-5p in the pancreas (both P < 0.05), and compared with the SAP group, the YCHT group had a significant reduction in the expression of lncRNA PVT1 and a significant increase in the expression of miRNA-30a-5p (both P < 0.05). Conclusion Cell autophagy and apoptosis mediated by lncRNA PVT1/miRNA-30a-5p may be a drug target for YCHT treatment of SAP, which provides experimental and theoretical bases for further development of the TCM prescription YCHT for the treatment of SAP. -
最新的全球癌症负担数据[1]显示,2020年的全球肝癌新发病例与新增死亡病例分别约90.57万例与83.02万例。这一年,肝癌在中国是发病率与死亡率均排列前十的肿瘤病种,新发肝癌患者约41万例,新增肝癌死亡患者约39万例[1],严重威胁人民的生命健康。肝细胞癌(HCC)占原发性肝癌的75%~85%[2]。经肝动脉化疗栓塞术(TACE)治疗有助于减轻化疗药物的全身毒副作用、提高局部肿瘤部位的药物浓度。目前TACE被国内外权威指南推荐用于不可切除肝细胞癌(uHCC)患者,是uHCC患者最常用的治疗方法之一[3-4]。TACE联合索拉非尼已被推荐用于中晚期HCC患者[3],最近一些文献报道了TACE联合抗程序性死亡受体1(programmed cell death protein 1,PD-1)及其配体(programmed cell death 1 ligand 1,PD-L1)单抗用于HCC患者的研究结果。TACE是否联合靶向药物、免疫检查点抑制剂是HCC患者TACE的重要预后因素。不过TACE中使用的化疗药物如何选择还不清晰,TACE联合靶向药物或PD-1/PD-L1单抗的临床研究数据不断更新,本文对近5年TACE单独、TACE联合靶向或PD-1/PD-L1单抗治疗uHCC患者的疗效与安全性研究作一综述,以期为临床治疗方案的选择提供参考。
1. 含不同化疗药物的TACE方案治疗uHCC患者的疗效与安全性
TACE是指将碘化油化疗药物乳剂或载药微球、补充栓塞剂(如聚乙烯醇颗粒)等经肿瘤供血动脉支注入的治疗[3],它是一种局部治疗。依据栓塞剂不同,TACE分为传统TACE(cTACE)与载药微球TACE(DEB-TACE)。DEB-TACE即预先加载化疗药物的药物洗脱微球栓塞治疗的方法。
HCC患者TACE治疗中常见化疗药物包括蒽环类、顺铂、丝裂霉素、氟尿嘧啶等,其中蒽环类常用的包括多柔比星、表柔比星、伊达比星等[5]。表 1总结了近5年关于不同化疗药物的TACE方案治疗uHCC患者的研究。2002年报道的两项重要RCT[6-7]分别证实了使用顺铂的cTACE与使用多柔比星的cTACE均比对症治疗显著改善uHCC患者的生存。ACE500研究[8]纳入日本uHCC患者,研究发现接受含顺铂的cTACE治疗组与含表柔比星的cTACE治疗组的中位总生存期(mOS)无显著差异,两组的客观缓解率(ORR)也无明显差异。另外这项研究显示顺铂组与表柔比星组的严重不良事件(AE)发生率相近。Ikeda等[9]发现含有米铂对比含有表柔比星的cTACE治疗uHCC患者的mOS无显著差异,不过米铂组的≥3级肝脏转氨酶升高发生率较表柔比星组低。该项研究未证实含米铂的TACE较含表柔比星的TACE治疗延长uHCC患者生存期,另外当前米铂仅在日本上市,含米铂的TACE适用于HCC患者也仅在日本获批,米铂在uHCC患者中的应用人群还比较局限。
表 1 近5年关于含不同化疗药物的TACE方案治疗uHCC患者的研究汇总Table 1. Summary of studies on the treatment of uHCC patients using TACE containing different chemotherapy drugs in recent five years研究者 受试者 研究设计 例数 试验组 对照组 mOS mPFS ORR ≥3级AE发生率 Aramaki O, 2021年[8] uHCC患者 Ⅱ/Ⅲ期
RCT研究455 cTACE(顺铂) cTACE
(表柔比星)2.9年vs 2.7年 未提供 65.3% vs 60.6% 49.8% vs 48.3 % Ikeda M, 2018年[9] uHCC患者 Ⅲ期RCT研究 247 cTACE(米铂) cTACE
(表柔比星)3.0年vs 3.1年 未提供 未提供 AST升高:39.5% vs 57.7 %,ALT升高:31.5% vs 53.7% Fu J, 2021年[11] uHCC患者,且对多柔比星耐药 前瞻性、RCT研究 160 cTACE(博来霉素) cTACE(多柔比星) 8.1个月vs 4.0个月 5.8个月vs 2.9个月 27.5% vs 7.5% AST升高:
5.0% vs 3.75 %Wang Y, 2018年[12] 中晚期HCC患者,且经≥2次cTACE治疗进展后 前瞻性、RCT研究 160 cTACE(博来霉素+吡柔比星+FOLFOX) cTACE(吡柔比星+FOLFOX) 8.1个月vs 4.0个月 5.8个月vs 2.9个月 27.5% vs 7.5% 两组均未出现严重治疗相关AE 注:FOLFOX,奥沙利铂联合氟尿嘧啶。 依据改良实体瘤疗效评价mRECIST标准,评价为疾病进展,而患者肝功能、体力状况等一般情况符合TACE要求,建议进行后续的TACE治疗[10]。Fu等[11]研究纳入了多柔比星耐药的uHCC患者,结果显示cTACE(博来霉素)治疗组的ORR、mOS、中位无进展生存期(mPFS)均显著高于cTACE(多柔比星)治疗组,且两组患者的术后并发症无显著差异。一致的是,一项研究[12]纳入中晚期HCC且经cTACE治疗进展后的患者,分别给予含有或不含有博来霉素的cTACE治疗(博来霉素组:博来霉素+吡柔比星+奥沙利铂+氟尿嘧啶;对照组:吡柔比星+奥沙利铂+氟尿嘧啶),结果显示含博来霉素组较不含博来霉素组有显著更长的mPFS与mOS,以及更高的ORR,该研究中未出现严重治疗相关AE。研究提示博来霉素的临床疗效和安全性较好,可作为uHCC患者cTACE治疗的二线用药选择。
目前TACE治疗中化疗药物的用量没有标准推荐,临床一般根据患者的体表面积、体力状况、肿瘤负荷、既往用药史、联合用药情况等选择用量[10]。一项研究[13]比较了含多柔比星不同剂量(低剂量组50 mg vs高剂量组100 mg)的TACE治疗中期HCC患者的疗效,共纳入28例受试者,研究发现两组患者的反应率与mOS均无显著差异,不过低剂量组比高剂量组患者的栓塞后发热与疼痛的持续时间均更短。
2. TACE联合靶向药物治疗uHCC患者的疗效与安全性
有研究[14]显示TACE联合靶向药物较TACE单独治疗uHCC患者的总生存期更高。研究[15]发现TACE治疗后HCC患者的血清血管内皮生长因子(VEGF)水平明显升高,这可能增加肿瘤复发或转移风险[16]。而索拉非尼、仑伐替尼、阿帕替尼等TKI,具有抑制VEGF受体VEGFR1、VEGFR2与VEGFR3的作用,TACE与TKI联合使用可能在HCC患者中发挥协同抗肿瘤作用。表 2总结了近5年关于TACE联合靶向药物治疗uHCC患者的前瞻性研究。
表 2 近5年关于TACE联合不同的靶向药物治疗uHCC患者的前瞻性研究汇总Table 2. Summary of prospective studies on TACE combined with different molecular targeted agents in the treatment of uHCC patients in recent five years研究者 受试者 研究设计 总样本量(例) 试验组 对照组 mOS/2年OS率 mPFS ORR Kudo M, 2020年[17] uHCC患者(BCLC A/B/C期) 多中心、RCT研究 156 TACE+索拉非尼 TACE 77.2% vs 64.6% 25.2个月vs 13.5个月 71.3% vs 61.8 % Xu X, 2018年[19] 肝癌患者(BCLC B/C期) RCT研究 156 TACE+索拉非尼 组2:TACE;
组3:索拉非尼未提供 未提供 28.6% vs 24.5% vs 22.2 % Ding X, 2021年[21] HCC合并门静脉瘤栓患者 RCT研究 64 TACE+仑伐替尼 TACE+索拉非尼 14.5个月vs 10.8个月 4.7个月vs 3.1个月 53.1% vs 25.0 % Lu W, 2017年[22] uHCC患者(BCLC B/C期) RCT研究 44 TACE+阿帕替尼 TACE 未提供 12.5个月vs 6.0个月 50.0% vs 27.3 % Turpin A, 2021年[27] uHCC患者(BCLC A/B期) RCT研究 78 TACE+舒尼替尼 TACE+安慰剂 25.0个月vs 20.5个月 9.1个月vs 5.5个月 26.0% vs 47.3 % Xu Q, 2018年[28] uHCC患者(BCLC A/B/C期) RCT研究 104 TACE+舒尼替尼 TACE+索拉非尼 9.2个月vs 13.2个月 6.9个月vs 10.2个月 37.3% vs 58.5 % Chan SL, 2017年[29] uHCC患者(BCLC B/C期) 单臂临床研究 50 TACE+阿昔替尼 无 18.8个月 8.4个月 68.2% 2.1 TACE联合索拉非尼
TACE联合索拉非尼已被中国临床肿瘤学会(CSCO)指南推荐用于HCC患者(CNLC分期Ⅱb期)[3]。TACTICS研究[17],是一项多中心随机对照试验(RCT)研究,发现TACE联合索拉非尼比TACE单独治疗uHCC患者的mPFS延长11.7个月。亚组分析显示mPFS的显著获益主要体现在巴塞罗那(BCLC)分期B期患者中,而在BCLC分期A期、C期的患者中未发现显著差异。一项研究[18]对START研究数据进一步分析发现,TACE联合索拉非尼较TACE单独治疗早中期uHCC患者的反应率与mOS均显著更高。相似的是,Xu等[19]研究发现,与TACE单用或索拉非尼单药治疗相比,TACE联合索拉非尼的治疗显著提高中晚期肝癌患者的疾病控制率(DCR)。一项Meta分析纳入了14项关于TACE联合索拉非尼治疗晚期HCC患者的疗效与安全性研究[20](受试者数n=1670),结果提示TACE联合索拉非尼较TACE单独治疗显著改善ORR、DCR及1年生存率,不过联合组发生的AE也更多,其中最常见的包括疲劳、手足皮肤反应和腹泻,这些AE都是可耐受的。综上分析,与TACE单独治疗比较,TACE联合索拉非尼治疗uHCC患者的生存获益更大,且不良反应可耐受。
2.2 TACE联合仑伐替尼
Ding等[21]比较了TACE联合仑伐替尼与TACE联合索拉非尼一线治疗伴有门静脉瘤栓的uHCC患者的疗效,结果显示TACE联合仑伐替尼组较TACE联合索拉非尼组的ORR与mPFS均显著更高,两组的mOS无显著差异,不过前一组较后一组的mOS有升高趋势,两组患者出现的AE及其发生率是相似的。该研究是单中心、小样本量的RCT研究,还有待进一步研究证实。
2.3 TACE联合阿帕替尼
一项单中心RCT研究[22]发现,TACE联合阿帕替尼较TACE单独治疗中晚期HCC患者的mPFS延长6.5个月(P<0.05)。之后多个回顾性研究探究了这一主题,一项Meta分析纳入了23项相关研究[23],结果表明与TACE单独治疗比较,TACE联合阿帕替尼治疗中晚期HCC患者显著提高1年生存率、ORR与DCR。一项回顾性研究[24]结果提示,TACE联合阿帕替尼对比TACE联合索拉非尼治疗晚期HCC患者的反应率与mOS均无显著差异。相似的是,Qiu等[25]的回顾性研究结果显示这两种治疗方法治疗晚期HCC患者的mOS差异不显著。另外,该研究显示TACE联合阿帕替尼较TACE联合索拉非尼治疗晚期HCC患者的mPFS更短。上述两项研究中的前者发现两种干预方式组最常见的不良反应是相似的,包括手足皮肤反应、腹泻、高血压、蛋白尿与疲劳[24]。而后者[25]发现3/4级AE在TACE联合阿帕替尼组比TACE联合索拉非尼组更常见,TACE联合阿帕替尼组的高血压、口腔或肛门溃疡和蛋白尿发生率较高,而TACE联合索拉非尼组的腹泻和脱发发生率较高。TACE联合阿帕替尼是否不劣于TACE联合索拉非尼有待前瞻性的大样本研究进一步证实。
2.4 TACE联合其他TKI
安罗替尼、舒尼替尼与阿昔替尼作用于抗VEGF/VEGFR途径,其联合TACE治疗可能提高TACE治疗在uHCC患者中的疗效,减少肿瘤转移风险。一项回顾性研究[26]结果显示,TACE联合安罗替尼较TACE单独治疗显著提高uHCC患者的ORR、mPFS、半年生存率及1年生存率,且治疗期间受试者未出现治疗相关的4级AE或死亡。Turpin等[27]研究发现,在uHCC患者中,TACE联合舒尼替尼较TACE单独治疗带来更长的mOS与mPFS。不过Xu等[28]的研究发现,与TACE联合索拉非尼比较,TACE联合舒尼替尼治疗uHCC患者未显示出优效性,而TACE联合索拉非尼组的mPFS与mOS显著更长、ORR也更高,虽然手足综合征在TACE联合索拉非尼组更常见。一项单臂临床研究[29]发现,接受TACE联合阿昔替尼治疗的uHCC患者的ORR、两年存活率、mOS分别为68.2%、43.7%、18.8个月。初步提示TACE联合阿昔替尼在uHCC中有较好的抗肿瘤活性与安全性。
3. TACE联合PD-1/PD-L1单抗治疗uHCC患者的疗效与安全性
3.1 TACE联合PD-1/PD-L1单抗
抗肿瘤免疫治疗主要包括免疫检查点抑制剂与细胞免疫治疗,前者包括PD-1/PD-L1单抗与CTLA-4单抗。卡瑞利珠单抗是一种PD-1单抗,一项RCT[30]纳入98例肿瘤负荷大的HCC患者,研究发现与TACE单独治疗比较,TACE联合卡瑞利珠单抗的治疗显著提高ORR(55.1% vs 22.5%)与DCR(77.6% vs 32.7%)。该研究中,所有的受试者均出现短暂的肝损伤、恶心呕吐、发热与腹痛,对症治疗后症状均得到缓解,两组的AE发生率无显著差异。一致的是,一项回顾性研究[31]纳入82例中晚期HCC患者,研究结果显示TACE联合卡瑞利珠单抗较TACE单独治疗显著提高ORR(61.9% vs 35.7%)与DCR(92.9% vs 69.1%),同时显著改善mPFS(12.9个月vs 8.4个月)与mOS(19.3个月vs 13.6个月)。该研究中联合组69.1%的患者出现与卡瑞利珠单抗相关的1级反应性毛细血管增生症,未出现严重AE。上述真实世界研究的数据提示TACE联合卡瑞利珠单抗较TACE单独治疗改善uHCC患者生存,且安全性可控。
3.2 TACE联合靶向药物及PD-1/PD-L1单抗
由于靶受体或下游信号的随机性变异,靶向治疗药物耐药仍然是uHCC治疗中的挑战性问题,TKI结合PD-1/PD-L1单抗的治疗对uHCC患者是一个有希望的策略[32]。在2022年美国临床肿瘤协会会议上,报告了一项关于TACE+仑伐替尼+卡瑞利珠单抗/信迪利单抗治疗HCC患者(BCLC分期B/C期)的前瞻性研究[33]的初步结果,显示在中位随访时间33.3周后,38例受试者转化为可手术切除的比率达50%,其中5例患者达完全病理缓解;48周的OS率与PFS率分别为96.4%与91.7%。该研究中57.9%受试者出现过3级治疗相关AE,未出现3级以上治疗相关AE。一项Meta分析纳入了该项研究与其他3个回顾性研究进行分析,结果提示TACE+仑伐替尼+抗PD-1单抗三联治疗较TACE单独治疗uHCC患者的手术转化率与ORR均更高,且三联治疗较双联治疗(TACE+放疗/TKI/肝动脉灌注化疗)在uHCC患者中的手术转化率(42% vs 19%)与ORR(71% vs 40%)均更高[34]。目前还有一些三联治疗的研究正在进行中,LEAP-012研究[35]将比较TACE+仑伐替尼+帕博利珠单抗的三联治疗与TACE+安慰剂治疗在中期uHCC患者中的疗效与安全性,另外TACE+贝伐珠单抗+阿替利珠单抗方案治疗中期HCC患者的单臂Ⅱ期临床研究正在开展[36]。
4. 总结
研究表明,使用含顺铂或多柔比星或表柔比星的cTACE均能改善uHCC患者生存,且出现严重不良AE的概率相似,尚不能确定cTACE使用其中哪种化疗药物对uHCC患者效果更佳,临床可综合评估患者肾功能、心脏功能、骨髓造血功能等指标之后选择TACE治疗中合适的化疗药物。虽然CSCO指南推荐TACE联合靶向治疗用于中晚期HCC患者,不过仅明确推荐TACE联合索拉非尼[3]。最近的研究发现TACE联合仑伐替尼较TACE联合索拉非尼给晚期HCC患者带来显著更高的ORR与mPFS,不过前一种较后一种治疗在延长mOS方面未显示出明显优效性,仅有提高的趋势。TACE联合靶向药物(仑伐替尼与阿帕替尼)治疗uHCC患者的前瞻性研究样本量均较小,未来开展扩大样本量的RCT将为临床提供更强的参考依据。初步研究提示TACE联合靶向药物及抗PD-1单抗的三联治疗对于uHCC患者似乎是一种更有前景的治疗策略,且安全性可控,不过疗效有待未来三联治疗的RCT结果进一步证实,继续深入阐明抗PD-1单抗影响靶向药物治疗uHCC患者疗效的作用机理也是需要的。
-
表 1 引物序列
基因 引物序列 lncRNA PVT1 R:5′-ACCAGGATATAAACCTAGTGATAAA-3′ F:5′-AAGCACCCAATGCAGAATAG-3′ GAPDH R:5′-ATGTTCCAGTATGACTCTA-3′ F:5′-CACCCCATTTGATGTTAG-3′ U6 R:5′-GGAACGATACAGAGAAGATTAGC-3′ F:5′-TGGAACGCTTCACGAATTTGCG-3′ 表 2 各组大鼠胰腺组织病理学评分以及血浆淀粉酶、TNFα、IL-1β水平的比较
组别 大鼠数(只) 病理学评分 淀粉酶(μmol/L) TNFα(ng/L) IL-1β(ng/L) SO组 10 1.667±0.577 2.259±0.935 4.444±1.684 2.129±0.931 SAP模型组 10 13.000±1.0001) 16.156±3.9161) 42.791±10.5021) 11.054±0.8501) YCHT治疗组 10 5.000±1.0002) 4.400±1.0892) 14.250±2.8212) 4.739±0.7272) F值 130.90 95.61 98.13 298.50 P值 <0.001 <0.001 <0.001 <0.001 注:与SO组相比,1)P<0.05;与SAP模型组相比,2)P<0.05。 表 3 各组自噬和凋亡标记蛋白相对表达量的比较
组别 大鼠数(只) LC-3Ⅱ/LC-3Ⅰ Beclin-1 XIAP Caspase-3 NF-κB SO组 10 0.360±0.049 0.767±0.017 0.227±0.002 0.264±0.035 0.701±0.007 SAP模型组 10 3.945±0.0931) 1.289±0.0131) 0.254±0.0041) 0.618±0.0351) 0.754±0.0001) YCHT治疗组 10 1.667±0.0622) 0.310±0.0212) 0.102±0.0162) 1.278±0.0612) 0.682±0.0142) F值 1498.0 2482.0 204.4 272.4 48.9 P值 <0.001 <0.001 <0.001 <0.001 <0.001 注:与SO组相比,1)P<0.05;与SAP模型组相比,2)P<0.05。 表 4 YCHT对胰腺组织中lncRNA-PVT1和miRNA-30a-5p相对表达量的影响
组别 大鼠数(只) lncRNA-PVT1 miRNA-30a-5p SO组 10 1.003±0.019 1.003±0.089 SAP模型组 10 1.626±0.0601) 0.021±0.0031) YCHT治疗组 10 0.253±0.1782) 3.404±0.1292) F值 119.7 1111.0 P值 <0.001 <0.001 注:与SO组相比,1)P<0.05;与SAP模型组相比,2)P<0.05。 -
[1] LANKISCH PG, APTE M, BANKS PA. Acute pancreatitis[J]. Lancet, 2015, 386(9988): 85-96. DOI: 10.1016/S0140-6736(14)60649-8 [2] XIANG H, ZHANG Q, QI B, et al. Chinese herbal medicines attenuate acute pancreatitis: Pharmacological activities and mechanisms[J]. Front Pharmacol, 2017, 8: 216. DOI: 10.3389/fphar.2017.00216 [3] SENDLER M, WEISS FU, GOLCHERT J, et al. Cathepsin B-mediated activation of trypsinogen in endocytosing macrophages increases severity of pancreatitis in mice[J]. Gastroenterology, 2018, 154(3): 704-718.e10. DOI: 10.1053/j.gastro.2017.10.018 [4] LIU Y, CHEN XD, YU J, et al. Deletion Of XIAP reduces the severity of acute pancreatitis via regulation of cell death and nuclear factor-κB activity[J]. Cell Death Dis, 2017, 8(3): e2685. DOI: 10.1038/cddis.2017.70 [5] LEVINE B. Cell biology: Autophagy and cancer[J]. Nature, 2007, 446(7137): 745-747. DOI: 10.1038/446745a [6] SU Z, YANG Z, XU Y, et al. MicroRNAs in apoptosis, autophagy and necroptosis[J]. Oncotarget, 2015, 6(11): 8474-8490. DOI: 10.18632/oncotarget.3523 [7] LI Y, JIANG J, LIU W, et al. microRNA-378 promotes autophagy and inhibits apoptosis in skeletal muscle[J]. Proc Natl Acad Sci U S A, 2018, 115(46): e10849-e10858. DOI: 10.1073/pnas.1803377115 [8] ZHOU W, XU J, WANG C, et al. miR-23b-3p regulates apoptosis and autophagy via suppressing SIRT1 in lens epithelial cells[J]. J Cell Biochem, 2019, 120(12): 19635-19646. DOI: 10.1002/jcb.29270 [9] QUINN JJ, CHANG HY. Unique features of long non-coding RNA biogenesis and function[J]. Nat Rev Genet, 2016, 17(1): 47-62. DOI: 10.1038/nrg.2015.10 [10] TIAN Q, LIU F, XU Z, et al. Evaluation of the chemical consistency of Yin-Chen-Hao-Tang prepared by combined and separated decoction methods using high-performance liquid chromatography and quadrupole time-of-flight mass spectrometry coupled with multivariate statistical analysis[J]. J Sep Sci, 2019, 42(9): 1664-1675. DOI: 10.1002/jssc.201800961 [11] XIANG H, WANG G, QU J, et al. Yin-Chen-Hao Tang attenuates severe acute pancreatitis in rat: An experimental verification of in silico network target prediction[J]. Front Pharmacol, 2016, 7: 378. DOI: 10.3389/fphar.2016.00378 [12] XIANG H, TAO X, XIA S, et al. Emodin alleviates sodium taurocholate-induced pancreatic acinar cell injury via MicroRNA-30a-5p-mediated inhibition of high-temperature requirement a/transforming growth factor beta 1 inflammatory signaling[J]. Front Immunol, 2017, 8: 1488. DOI: 10.3389/fimmu.2017.01488 [13] LEE PJ, PAPACHRISTOU GI. New insights into acute pancreatitis[J]. Nat Rev Gastroenterol Hepatol, 2019, 16(8): 479-496. DOI: 10.1038/s41575-019-0158-2 [14] HAHM KB, KIM JH, YOU BM, et al. Induction of apoptosis with an extract of Artemisia asiatica attenuates the severity of cerulein-induced pancreatitis in rats[J]. Pancreas, 1998, 17(2): 153-157. DOI: 10.1097/00006676-199808000-00007 [15] IWAHASHI K, HIKITA H, MAKINO Y, et al. Autophagy impairment in pancreatic acinar cells causes zymogen granule accumulation and pancreatitis[J]. Biochem Biophys Res Commun, 2018, 503(4): 2576-2582. DOI: 10.1016/j.bbrc.2018.07.018 [16] GUKOVSKAYA AS, PERKINS P, ZANINOVIC V, et al. Mechanisms of cell death after pancreatic duct obstruction in the opossum and the rat[J]. Gastroenterology, 1996, 110(3): 875-884. DOI: 10.1053/gast.1996.v110.pm8608898 [17] PARZYCH KR, KLIONSKY DJ. An overview of autophagy: Morphology, mechanism, and regulation[J]. Antioxid Redox Signal, 2014, 20(3): 460-473. DOI: 10.1089/ars.2013.5371 [18] WANG S, DING WX. Does autophagy promote or protect against the pathogenesis of pancreatitis?[J]. Gastroenterology, 2018, 155(4): 1273-1274. DOI: 10.1053/j.gastro.2018.02.046 [19] KONDO Y, KANZAWA T, SAWAYA R, et al. The role of autophagy in cancer development and response to therapy[J]. Nat Rev Cancer, 2005, 5(9): 726-734. DOI: 10.1038/nrc1692 [20] YANG Y, HUANG Q, LUO C, et al. MicroRNAs in acute pancreatitis: From pathogenesis to novel diagnosis and therapy[J]. J Cell Physiol, 2020, 235(3): 1948-1961. DOI: 10.1002/jcp.29212 [21] XIANG H, TAO X, XIA S, et al. Targeting MicroRNA function in acute pancreatitis[J]. Front Physiol, 2017, 8: 726. DOI: 10.3389/fphys.2017.00726 [22] ABREU FB, LIU X, TSONGALIS GJ. miRNA analysis in pancreatic cancer: The Dartmouth experience[J]. Clin Chem Lab Med, 2017, 55(5): 755-762. [23] QUAN X, LI X, YIN Z, et al. p53/miR-30a-5p/ SOX4 feedback loop mediates cellular proliferation, apoptosis, and migration of non-small-cell lung cancer[J]. J Cell Physiol, 2019, 234(12): 22884-22895. DOI: 10.1002/jcp.28851 [24] KIM JW, YOU YH, JUNG S, et al. miRNA-30a-5p-mediated silencing of Beta2/NeuroD expression is an important initial event of glucotoxicity-induced beta cell dysfunction in rodent models[J]. Diabetologia, 2013, 56(4): 847-855. DOI: 10.1007/s00125-012-2812-x [25] ZHOU L, JIA S, DING G, et al. Down-regulation of miR-30a-5p is associated with poor prognosis and promotes chemoresistance of gemcitabine in pancreatic ductal adenocarcinoma[J]. J Cancer, 2019, 10(21): 5031-5040. DOI: 10.7150/jca.31191 [26] YANG X, BAI F, XU Y, et al. Intensified Beclin-1 Mediated by low expression of Mir-30a-5p promotes chemoresistance in human small cell lung cancer[J]. Cell Physiol Biochem, 2017, 43(3): 1126-1139. DOI: 10.1159/000481754 [27] XU X, JIN S, MA Y, et al. miR-30a-5p enhances paclitaxel sensitivity in non-small cell lung cancer through targeting BCL-2 expression[J]. J Mol Med (Berl), 2017, 95(8): 861-871. DOI: 10.1007/s00109-017-1539-z [28] YAO RW, WANG Y, CHEN LL. Cellular functions of long noncoding RNAs[J]. Nat Cell Biol, 2019, 21(5): 542-551. DOI: 10.1038/s41556-019-0311-8 期刊类型引用(7)
1. 郭玉祥,王茂森,刘中原,张旭东,马鹏飞,王向坤,李仁锋. 肝细胞癌合并胆管癌栓的诊疗进展. 临床肝胆病杂志. 2025(02): 359-364 .  本站查看
本站查看2. 吴园园,李晨露,陈燕,曹梦妲,邵华. 替雷利珠联合仑伐替尼治疗中晚期肝癌的临床疗效分析. 中国临床药理学与治疗学. 2025(03): 392-397 .  百度学术
百度学术3. 张楚悦,史家宁,王明达,吴寒,史立军,杨田. 微球和纳米颗粒在经颈动脉化疗栓塞治疗肝细胞癌中的应用. 临床肝胆病杂志. 2024(04): 816-821 .  本站查看
本站查看4. 陈萍. 贝伐珠单抗、奥沙利铂联合信迪利单抗治疗结直肠癌临床效果及对肿瘤标志物、免疫功能的影响. 交通医学. 2024(01): 40-42+45 .  百度学术
百度学术5. 徐静,丁哲,徐颖. 基于TumorFisher CTC检测探讨PD-1/PD-L1免疫抑制剂治疗肝细胞癌的效果. 肝脏. 2024(10): 1184-1188 .  百度学术
百度学术6. 张琳,冯晓彬,黄鑫,梁子威,何作祥,卢倩,董家鸿. 钇-90微球选择性内放射治疗在肝癌降期转化移植中的应用进展. 中华消化外科杂志. 2024(12): 1566-1570 .  百度学术
百度学术7. 陈治莉,毛创杰,邓杨,贺秋凤,胡蓉. 术前血清γ-谷氨酰转肽酶水平对老年肝细胞癌患者行肝动脉化疗栓塞术后预后的影响分析. 老年医学与保健. 2023(05): 901-905 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 6634 KB)
PDF下载 ( 6634 KB)

 下载:
下载:
 下载:
下载:
 百度学术
百度学术

