基于术前人B-淋巴细胞趋化因子1检测的肝细胞癌患者肝切除术后复发预测模型的构建及分析
DOI: 10.3969/j.issn.1001-5256.2021.04.020
Construction and analysis of a predictive model for posthepatectomy recurrence in patients with hepatocellular carcinoma based on preoperative CXCL13 measurement
-
摘要:
目的 探究与肝细胞癌患者肝切除术后复发相关的血清学指标,构建复发预后模型以评估患者是否适合采用姑息性肝切除手术。 方法 共纳入了2009年2月—2013年7月于郑州大学附属肿瘤医院接受肝切除术并进行随访的肝细胞癌患者111例。收集患者的基本资料,根据患者在随访期间内是否复发分为复发组和未复发组。正态分布的计量资料两组间比较采用t检验;非正态分布的计量资料两组间比较采用Wilcoxon秩和检验。计数资料组间比较采用χ2检验。Kaplan-Meier法绘制生存曲线,log- rank检验比较差异。单因素与多因素分析采用Cox回归分析,采用受试者工作特征曲线(ROC曲线)判断预测效能。 结果 Kaplan-Meier生存曲线显示AFP、ALP、GGT、FIB低值组及人B-淋巴细胞趋化因子1(CXCL13) 高值组患者的中位复发时间更长(P值均<0.05)。AFP[HR(95%CI)=1.69(1.03~2.79),P=0.039]、GGT[HR(95%CI)=1.89(1.14~3.14),P=0.014]和CXCL13[HR(95%CI)=0.54(0.33~0.89),P=0.015]为与肝切除术后复发相关的独立因素。基于其构成的预后指数:PI=0.526×AFP+0.637×GGT-0.616×CXCL13,预测姑息性切除术后0~3个月内复发的ROC曲线下面积(AUC)为0.87,灵敏度为93.75%,特异度为63.64%,但对0~6个月(AUC=0.68)及更长时间的预测效能明显下降,对姑息性切除的复发预测效能显著高于根治性切除。 结论 基于CXCL13、AFP和GGT构建的预后模型可用于评估患者采用姑息性肝切除术后的早期复发风险,有助于临床医生依据患者获益情况作出诊疗决策。 Abstract:Objective To investigate the serological markers associated with posthepatectomy recurrence in patients with hepatocellular carcinoma, and to establish a prognostic model to evaluate whether palliative hepatectomy is suitable for such patients. Methods A total of 111 patients with hepatocellular carcinoma who underwent hepatectomy in the Affiliated Cancer Hospital of Zhengzhou University from February 2009 to July 2013 and received follow-up were enrolled. Basic clinical data were collected and the patients were divided into recurrence group and non-recurrence group according to whether recurrence was observed during follow-up. The t-test was used for comparison of normally distributed continuous data between two groups and the Wilcoxon rank sum test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test was used for comparison of categorical data between two groups. Survival curves were plotted using the Kaplan-Meier method, and survival differences were analyzed using the log-rank test. A Cox regression analysis was used to perform univariate and multivariate analyses, and the area under the ROC curve (AUC) was used to evaluate prediction efficiency. Results The Kaplan-Meier survival curves showed that the patients with low alpha-fetoprotein (AFP), alkaline phosphatase, gamma-glutamyl transpeptidase (GGT), and fibrinogen and high CXCL13 had a longer median time to recurrence (P < 0.05). AFP (hazard ratio [HR][95%CI]=1.69(1.03~2.79), P=0.039), GGT (HR[95%CI]=1.89(1.14~3.14), P=0.014), and CXCL13 (HR[95%CI]=0.54(0.33~0.89), P=0.015) were independent factors associated with posthepatectomy recurrence. The prognostic index PI=0.526×AFP+0.637×GGT-0.616×CXCL13 established based on these factors had an AUC of 0.87, a sensitivity of 93.75%, and a specificity of 63.64% in predicting recurrence within 0-3 months after palliative hepatectomy, with a significant reduction in prediction efficiency for recurrence within 0-6 months (AUC=0.68) or a longer period of time. The recurrence prediction efficiency of this model for palliative hepatectomy was significantly higher than that for radical resection. Conclusion The prognostic model established based on CXCL13, AFP, and GGT can be used to evaluate the risk of early recurrence after palliative hepatectomy and thus helps clinicians to make diagnosis and treatment decisions based on patients' benefits. -
Key words:
- Carcinoma, Hepatocellular /
- Hepatectomy /
- Recurrence /
- Forecasting /
- Models, Statistical
-
肝细胞癌(简称肝癌)占全球癌症发病率的第5位、男性癌症死亡率的第2位[1]以及我国癌症发病率的第4位,严重危害人民的生命与健康。近年来,随着射频消融、经肝动脉化学栓塞等非手术治疗技术的引入,肝癌的临床治疗手段有了一定的进展。患者的术后复发与多种因素相关,已有研究者[2]将肿瘤因素、肝功能状态、手术方式等纳入肝癌复发模型。目前,姑息性手术切除仍是中晚期肝癌的选项之一,但由于患者术后复发时间短、预后差,对部分患者是否应行姑息手术存在争议。本研究对接受肝切除术的患者进行分析,试图从术前的血清学检查中发现与肝癌术后复发相关的指标,以便在术前对患者预后进行评估。人B-淋巴细胞趋化因子1(C-X-C Motif Chemokine Ligand 13,CXCL13)是趋化因子家族中的一员,主要与人体细胞表面的G蛋白耦联受体结合,主要分布在肝脏、淋巴结等器官组织中。CXCL13作为CXCR5(C-X-C chemokine receptor type 5)的唯一配体,是人类免疫系统的重要组成部分[3],其在肿瘤患者中的水平高于健康人群和慢性乙型肝炎患者[4]。本实验室前期研究[5]发现,在低分化的肿瘤组中,CXCL13和CXCR5在肿瘤组织中高于癌旁组织,并且证实了CXCL13水平与无复发生存相关而与总生存无关。也有研究[6]报道CXCL13基因缺失的大肠癌患者具有更高的复发风险。但是,CXCL13对术后肝癌患者预后的意义并不清楚。本研究对接受肝切除术后的肝细胞癌患者的术前血清学指标进行分析,尝试建立可用于术前评估患者能否从姑息性肝切除中获益的评价体系,为开展个体化的治疗选择提供帮助。
1. 资料与方法
1.1 研究对象
回顾性纳入2009年2月22日—2013年7月13日在郑州大学附属肿瘤医院行肝切除术且完成随访的111例患者,队列失访率为11.81%,其基本信息已在实验室前期文章汇总进行描述[5]。纳入标准:接受肝切除术并最终病理诊断为肝细胞癌的患者。排除标准:(1)临床资料缺失严重;(2)肝切除术前接受其他方式治疗。对符合纳入标准的患者采用门诊查询、电话咨询等方式进行随访。出院3个月后患者复查时进行首次门诊查询,未复查者进行电话咨询,出院后1年内每3个月随访1次,2~3年内每6个月随访1次,4~5年内每年随访1次,询问其生存状态,复发情况。肝癌复发定义为影像学检查发现肿瘤灶。随访至2014年7月25日,在随访期间内,当患者出现复发或死亡时,记录其无复发生存时间及复发状态。
1.2 研究方法
从电子病历中收集患者的基本状况:性别、年龄、肿瘤数目、肿瘤大小、肝硬化情况、Child分级、BCLC分期以及术前最后1次血清学检测数据[AFP、总胆汁酸(TBA)、TBil、ALT、AST、前白蛋白(PA)、RBC、Alb、Glo、白球比(A/G)、ALP、GGT、淋巴细胞/单核细胞比值(LMR)、中性粒细胞/淋巴细胞比值(NLR)、PLT、PT、国际标准化比值(INR)、纤维蛋白原(FIB)等]。患者手术麻醉前抽取血液,1500 r/min离心5 min,收集血清,冻存至-80 ℃,转运过程中使用干冰运输。2014年于北京大学采用ProcartaPlexTM Multiplex Immunoassays (Affymetrix,圣地亚哥,加州,美国)试剂盒检测患者血清CXCL13水平。根据2011年原发性肝癌诊疗规范[7]中根治性切除的Ⅲ级标准,将患者的手术方式分为姑息性切除和根治性切除。
1.3 伦理学审查
本研究经北京大学生物医学伦理委员会批准,批号:IRB00001052-12088,所有参加者均获得书面知情同意。
1.4 统计学方法
采用SPSS 24.0统计软件进行数据分析。正态分布的计量资料以x±s表示,两组间比较采用t检验;非正态分布的计量资料以M(P25~P75)表示,两组间比较采用Wilcoxon秩和检验。计数资料组间比较采用χ2检验。绘制Kaplan-Meier生存曲线,log-rank检验比较差异,单因素与多因素分析采用Cox回归分析,采用向前逐步法进行变量筛选(引入:P<0.05,剔除:P>0.1),检验水准α=0.05。经MedCalc进行受试者工作特征曲线(ROC曲线)分析,ROC曲线下面积(AUC)判断预测效能。P<0.05为差异有统计学意义。
2. 结果
2.1 肝切除术后复发和术后未复发患者的术前指标对比
111例患者在随访期间发生术后复发71例(复发组),未复发40例(未复发组),随访人年为69.29年。复发组的术前AFP、TBil、GGT的水平高于未复发组,BCLC分期存在差异(P值均<0.05)(表 1)。
表 1 肝切除术后未复发组和复发组患者术前指标比较指标 未复发组(n=40) 复发组(n=71) 统计值 P值 男/女(例) 33/7 57/14 χ2=0.82 0.775 年龄(岁) 53.83±11.05 54.85±10.11 t=-0.36 0.717 肝硬化(无/有,例)1) 6/34 7/62 χ2=0.57 0.655 肿瘤数目(1个/≥2个,例) 33/7 57/14 χ2=0.98 0.775 肿瘤大小(>5 cm/≤5 cm,例)1) 20/16 42/27 χ2=0.28 0.599 AFP(ng/μl) 14.82(2.43~1000.00) 329.60(15.37~1210.00) Z=-2.74 0.006 TBA(μmol/L) 12.20(7.03~17.00) 12.20(8.40~20.70) Z=-0.31 0.760 TBil(μmol/L) 13.65(8.60~17.25) 15.00(10.80~19.80) Z=-2.18 0.029 ALT(U/L) 40.50(24.25~62.50) 31.00(24.00~49.00) Z=-1.27 0.204 AST(U/L) 35.50(25.25~60.75) 42.00(30.00~63.00) Z=-1.59 0.111 PA(mg/L) 160.50(125.65~197.73) 160.50(129.00~185.00) Z=-1.10 0.269 Alb(g/L) 40.10(35.50~44.10) 40.50(36.80~44.20) Z=-0.35 0.729 Glo(g/L) 31.08±7.18 32.34±5.13 t=-1.07 0.287 A/G 1.35(1.08~1.54) 1.25(1.06~1.48) Z=-0.83 0.407 FIB(g/L) 2.80(2.25~3.20) 2.85(2.45~3.62) Z=-1.15 0.250 ALP(U/L) 94.50(68.00~136.25) 108.00(80.00~140.00) Z=-1.04 0.298 GGT(U/L) 46.00(30.20~81.25) 90.00(39.00~149.00) Z=-2.69 0.007 NLR 2.37(1.58~3.16) 2.46(1.75~3.16) Z=-0.08 0.936 LMR 4.55±1.94 4.37±1.68 t=0.50 0.617 RBC(×1012/L) 4.40(4.11~4.76) 4.40(3.99~4.72) Z=-1.35 0.892 CXCL13(pg/ml) 114.99(55.86~149.29) 65.91(22.14~142.82) Z=-1.43 0.153 PT(s) 12.90(11.85~13.63) 12.90(12.20~14.10) Z=-1.21 0.226 INR 1.09(1.01~1.14) 1.09(1.03~1.20) Z=-0.88 0.380 PLT(×109/L) 147.00(117.00~182.75) 147.00(85.00~204.00) Z=-0.40 0.692 Child分级A/B(例)1) 34/2 59/3 χ2=0.202 0.904 BCLC分期A/B/C(例)1) 30/6/1 34/14/21 χ2=13.17 0.001 注:1)部分病例的部分数据缺失,亚组间的数目存在差异。 2.2 不同水平的指标对术后复发的影响
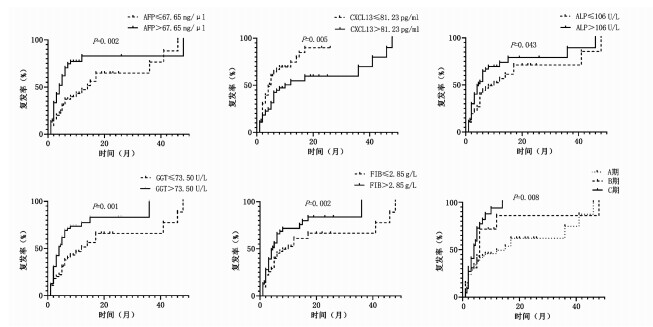
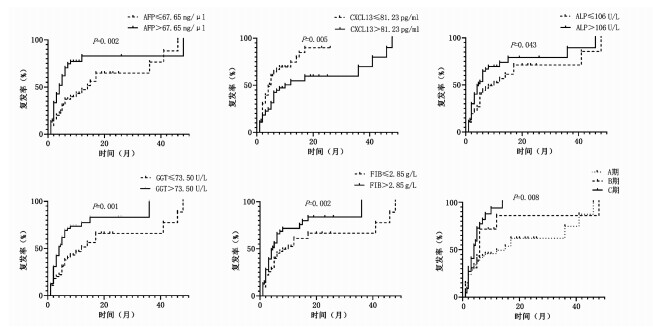
将连续性变量按照中位数分为高值组和低值组,AFP、FIB、ALP、GGT、CXCL13、BCLC分期不同分组之间的中位复发时间的差异具有统计学意义(P值均<0.05)(表 2)。Kaplan-Meier曲线显示AFP、ALP、GGT、FIB低值组及CXCL13高值组的中位复发时间更长,而随着BCLC分期的进展,中位复发时间缩短(图 1)。
表 2 各指标高低两组的中位复发时间指标 例数 中位复发时间(月) χ2值 P值 指标 例数 中位复发时间(月) χ2值 P值 性别(例) 0.03 0.874 A/G(例) 3.08 0.079 男 90 6.00 >1.30 56 10.00 女 21 5.00 ≤1.30 55 5.00 年龄(例) 0.39 0.532 FIB(例) 5.34 0.021 >56.00岁 48 6.00 >2.85 g/L 48 4.50 ≤56.00岁 63 5.00 ≤2.85 g/L 63 10.00 肝硬化(例) 0.05 0.816 ALP(例) 4.08 0.043 有 96 6.00 >106.00 U/L 54 4.50 无 13 6.00 ≤106.00 U/L 57 10.00 肿瘤数目(例) 0.39 0.532 GGT(例) 10.20 0.001 >1个 90 5.00 >73.50 U/L 55 4.00 1个 21 5.00 ≤73.50 U/L 56 12.00 肿瘤大小(例) 2.21 0.137 NLR(例) 0.03 0.856 >5 cm 62 5.00 >2.44 55 6.00 ≤5 cm 43 8.00 ≤2.44 56 6.00 AFP(例) 10.04 0.002 LMR(例) 0.12 0.727 >67.65 ng/μl 54 4.00 >4.43 55 6.00 ≤67.65 ng/μl 57 14.00 ≤4.43 56 6.00 TBA(例) 0.27 0.601 RBC(例) 0.89 0.345 >12.20 μmol/L 43 6.00 >4.40×1012/L 49 5.00 ≤12.20 μmol/L 68 6.00 ≤4.40×1012/L 62 6.00 TBil(例) 1.73 0.188 CXCL13(例) 7.90 0.005 >14.35 μmol/L 55 6.00 >81.23 pg/ml 55 10.00 ≤14.35 μmol/L 56 6.00 ≤81.23 pg/ml 56 4.50 ALT(例) 0.91 0.339 PT(例) 2.28 0.131 >35.50 U/L 55 7.00 >12.90 s 50 8.00 ≤35.50 U/L 56 6.00 ≤12.90 s 61 5.00 AST(例) 3.66 0.056 INR(例) 1.41 0.235 >39.50 U/L 55 5.00 >1.09 45 5.00 ≤39.50 U/L 56 12.00 ≤1.09 66 6.00 PA(例) 0.00 0.962 PLT(例) 0.28 0.596 >160.50 mg/L 47 6.00 >147.00×109/L 52 6.00 ≤160.50 mg/L 64 6.00 ≤147.00×109/L 59 6.00 Alb(例) 1.04 0.307 Child分级(例) 0.66 0.719 >40.50 g/L 55 5.00 A级 93 6.00 ≤40.50 g/L 56 8.00 B级 5 3.00 Glo(例) 0.98 0.320 BCLC分期(例) 12.36 <0.001 >31.50 g/L 52 5.00 A 64 15.00 ≤31.50 g/L 59 7.00 B 20 6.00 C 22 4.00 2.3 与术后复发相关联的因素及预后模型构建
将单因素分析中有差异的变量纳入多因素Cox回归分析,显示AFP、GGT、CXCL13为复发的独立因素[风险比(HR)(95%CI)分别为1.69(1.03~2.79)、1.89(1.14~3.14)、0.540(0.33~0.89),P值分别为0.039、0.014、0.015](模型系数Omnibus检验,χ2=4.323,P=<0.001)(表 3)。以此3个参数构建复发模型的风险函数表达式为h(t)=h0texp(0.526×AFP+0.637×GGT-0.616×CXCL13), 预后指数(Prognostic Index,PI)为:PI=0.526×AFP+0.637×GGT -0.616×CXCL13。PI最低值为-0.62,最高值为1.16,在全部患者中的中位数为0.53(0.00~0.55)。
表 3 与肝癌复发相关的单因素分析指标 β HR (95%CI) P值 AFP(ng/μl) 0.734 2.08(1.28~3.39) 0.003 TBA(μmol/L) 0.123 1.13(0.70~1.83) 0.618 TBil(μmol/L) 0.302 1.35(0.84~2.17) 0.211 ALT(U/L) -0.220 0.80(0.50~1.29) 0.803 AST(U/L) 0.436 1.55(0.96~2.48) 0.070 PA(mg/L) -0.011 0.99(0.61~1.61) 0.964 Alb(g/L) -0.235 0.79(0.49~1.27) 0.332 Glo(g/L) 0.231 1.26(0.78~2.04) 0.347 A/G -0.404 0.67(0.42~1.08) 0.096 FIB(g/L) 0.530 1.17(1.05~2.74) 0.030 ALP(U/L) 0.461 1.59(0.99~2.54) 0.056 GGT(U/L) 0.749 2.12(1.29~3.47) 0.003 NLR 0.168 1.18(0.68~2.06) 0.550 LMR 0.100 1.10(0.68~1.78) 0.683 RBC(×1012/L) 0.216 1.24(0.78~1.99) 0.369 CXCL13(pg/ml) -0.666 0.51(0.31~0.84) 0.009 PT(s) 0.345 1.41(0.88~2.26) 0.152 INR 0.274 1.32(0.82~2.12) 0.259 PLT(×109/L) 0.123 1.13(0.70~1.82) 0.614 2.4 预测模型的ROC曲线
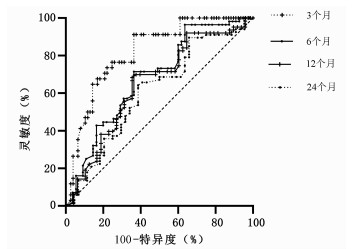
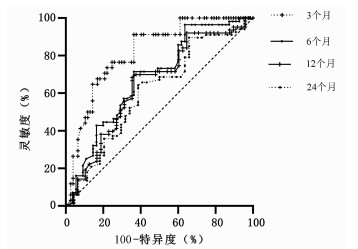
本随访研究队列患者肝切除术后3~24个月的复发率为30.63%~60.36%。经ROC曲线评估上述Cox的预测模型的生存分析函数(表 4),结果显示该模型对3个月复发风险的预测效能最高,AUC为0.82,灵敏度和特异度分别为91.18%和63.64%(图 2)。2.5 Cox模型对姑息和根治术后复发预测效能的比较进一步将患者按照姑息性切除和根治性切除分组,分别分析模型在姑息性切除和根治性切除后不同时间段的预测效能。结果显示该模型在各个时间段内对姑息性切除的预测效能均优于对根治性切除的预测效能(表 5)。该模型对姑息性切除0~3个月的AUC最高为0.87,其灵敏度和特异度分别为93.75%和70.00%。
表 4 Cox模型预测不同时间段复发情况的AUC、灵敏度及特异度时间 复发率 AUC(95%CI) 灵敏度 特异度 0~3个月 30.63% 0.82(0.74~0.89) 91.18% 63.64% 0~6个月 50.45% 0.68(0.59~0.77) 71.43% 63.64% 0~12个月 56.76% 0.65(0.55~0.74) 68.25% 64.58% 0~24个月 60.36% 0.61(0.51~0.70) 64.18% 61.36% 表 5 Cox模型预测姑息性切除和根治性切除不同时间段复发情况的AUC、灵敏度及特异度方法 例数 时间 AUC(95%CI) 灵敏度 特异度 姑息性切除 72 0~3个月 0.87(0.77~0.94) 93.75% 70.00% 0~6个月 0.72(0.60~0.82) 72.92% 66.67% 0~12个月 0.68(0.56~0.79) 69.81% 68.42% 0~24个月 0.63(0.51~0.74) 92.86% 37.50% 根治性切除 39 0~3个月 0.50(0.34~0.66) 100.00% 32.43% 0~6个月 0.57(0.40~0.72) 62.50% 61.29% 0~12个月 0.54(0.31~0.70) 50.00% 72.41% 0~24个月 0.51(0.35~0.67) 0 82.14% 3. 讨论
本研究对111例肝细胞癌肝切除术后随访患者的术前血清学指标进行Cox多因素分析,最终发现AFP、GGT、CXCL13是肝切除术后复发的独立影响因素,其中AFP、GGT为独立危险因素,CXCL13为独立保护因素。这3个指标分别从肿瘤标志物、炎症和宿主固有免疫3个方面预示着肝切除术后的复发风险。利用Cox建立复发预测模型,发现其对姑息性切除术后短期复发(0~3个月)预测的AUC最高,可帮助临床医师在一定程度上评估患者能否从姑息性切除术中获益。
有研究[4, 8-9]报道,随着肝癌临床分期的进展,患者血清CXCL13的浓度逐渐升高,且CXCL13水平与ALT、肿瘤大小、Child评分呈正相关。血清CXCL13水平在肝癌患者中高于健康人群,在低分化组的肝癌组织中高于高分化组,CXCL13可以募集CXCR5阳性的滤泡辅助性T淋巴细胞和B淋巴细胞,促进IL-21和IL-12的分泌,对肿瘤产生抑制作用[5, 9],也可以诱发外周器官中的次级淋巴组织形成,参与机体的免疫[10-11]。本研究中显示高水平的CXCL13患者拥有更好的预后,这可能与CXCL13作为免疫趋化因子参与机体的抗肿瘤免疫相关。
肝癌的复发分为早期复发和晚期复发,通常以术后2年为截止点[12]。本研究结果显示,其预测肝癌切除术后3个月内复发的效能最高,随着手术后生存时间的延长,该模型的预测能力下降。而考虑到接受姑息性切除的肝癌患者术后短期复发的患者可能更多,因此,根据患者的手术切除方式将其进一步分为姑息性切除和根治性切除,分别对该预测模型进行验证。结果显示该模型在不同时间段内预测姑息性切除复发的效果均优于根治性切除,同时对0~3个月复发预测的灵敏度最高,由此可见,该预测模型对术后短期复发的预测能力最佳,可用于预测姑息性切除术后的早期复发情况。
陈凯等[13]根据肿瘤因素建立了肝癌术后早期复发风险的模型,包含肿瘤直径、肿瘤数目、血管侵犯和肝外侵犯。周正等[2]则将手术方式、肿瘤因素和肝功能状态一同考虑,将手术器械、肝硬化、肿瘤直径、肿瘤数目、肿瘤分化程度等一同构建模型,其AUC为0.74,特异度为85.3%,灵敏度为52.3%。Pan等[14]将HBsAg同肿瘤数目、癌栓、分化程度、血管浸润等构建了列线图,并在验证队列中得到证实。叶颖剑等[15]在血清AFP阴性的肝细胞癌患者中发现肿瘤直径、肿瘤包膜和血管侵袭性与患者的复发相关。肝癌的复发与机体状态、肿瘤因素及手术因素等多种因素相关[2, 15-17]。不同于以往研究,本研究主要是基于CXCL13及其他术前血清学指标对患者的复发情况进行分析,具有简单易行的特点;特别是针对行肝癌姑息性切除的患者,可以从一定程度上提示术后复发风险,对0~3个月姑息手术切除患者的灵敏度高达93.75%,有助于临床医师进行临床诊疗决策。原发性肝癌诊疗规范(2019年版)[18]的根治性切除界定是根据术中和术后的判断标准进行的,不再有姑息性切除的描述。但术前基于血清学指标对预后的判定,对是否选择外科手术治疗仍有价值。但本研究也存在着一定局限性,如仅基于血清学指标进行分析的可靠性问题及样本量较小等,仍需大样本数据进行进一步分析及验证。
总之,本研究证实通过检测简单易行的术前血清学指标可以对肝细胞癌姑息性切除术后患者的早期复发情况进行较为准确的预测,对于早期复发风险较高的患者,在选择相关手术治疗时应更为审慎,可尝试采用其他联合或代替治疗的疗法。
志谢: 感谢所有参与本研究的患者及其家人的理解和支持,感谢姬旭慧、刘媛、谢霄梦等参与采集样本及随访的研究生同学。 -
表 1 肝切除术后未复发组和复发组患者术前指标比较
指标 未复发组(n=40) 复发组(n=71) 统计值 P值 男/女(例) 33/7 57/14 χ2=0.82 0.775 年龄(岁) 53.83±11.05 54.85±10.11 t=-0.36 0.717 肝硬化(无/有,例)1) 6/34 7/62 χ2=0.57 0.655 肿瘤数目(1个/≥2个,例) 33/7 57/14 χ2=0.98 0.775 肿瘤大小(>5 cm/≤5 cm,例)1) 20/16 42/27 χ2=0.28 0.599 AFP(ng/μl) 14.82(2.43~1000.00) 329.60(15.37~1210.00) Z=-2.74 0.006 TBA(μmol/L) 12.20(7.03~17.00) 12.20(8.40~20.70) Z=-0.31 0.760 TBil(μmol/L) 13.65(8.60~17.25) 15.00(10.80~19.80) Z=-2.18 0.029 ALT(U/L) 40.50(24.25~62.50) 31.00(24.00~49.00) Z=-1.27 0.204 AST(U/L) 35.50(25.25~60.75) 42.00(30.00~63.00) Z=-1.59 0.111 PA(mg/L) 160.50(125.65~197.73) 160.50(129.00~185.00) Z=-1.10 0.269 Alb(g/L) 40.10(35.50~44.10) 40.50(36.80~44.20) Z=-0.35 0.729 Glo(g/L) 31.08±7.18 32.34±5.13 t=-1.07 0.287 A/G 1.35(1.08~1.54) 1.25(1.06~1.48) Z=-0.83 0.407 FIB(g/L) 2.80(2.25~3.20) 2.85(2.45~3.62) Z=-1.15 0.250 ALP(U/L) 94.50(68.00~136.25) 108.00(80.00~140.00) Z=-1.04 0.298 GGT(U/L) 46.00(30.20~81.25) 90.00(39.00~149.00) Z=-2.69 0.007 NLR 2.37(1.58~3.16) 2.46(1.75~3.16) Z=-0.08 0.936 LMR 4.55±1.94 4.37±1.68 t=0.50 0.617 RBC(×1012/L) 4.40(4.11~4.76) 4.40(3.99~4.72) Z=-1.35 0.892 CXCL13(pg/ml) 114.99(55.86~149.29) 65.91(22.14~142.82) Z=-1.43 0.153 PT(s) 12.90(11.85~13.63) 12.90(12.20~14.10) Z=-1.21 0.226 INR 1.09(1.01~1.14) 1.09(1.03~1.20) Z=-0.88 0.380 PLT(×109/L) 147.00(117.00~182.75) 147.00(85.00~204.00) Z=-0.40 0.692 Child分级A/B(例)1) 34/2 59/3 χ2=0.202 0.904 BCLC分期A/B/C(例)1) 30/6/1 34/14/21 χ2=13.17 0.001 注:1)部分病例的部分数据缺失,亚组间的数目存在差异。 表 2 各指标高低两组的中位复发时间
指标 例数 中位复发时间(月) χ2值 P值 指标 例数 中位复发时间(月) χ2值 P值 性别(例) 0.03 0.874 A/G(例) 3.08 0.079 男 90 6.00 >1.30 56 10.00 女 21 5.00 ≤1.30 55 5.00 年龄(例) 0.39 0.532 FIB(例) 5.34 0.021 >56.00岁 48 6.00 >2.85 g/L 48 4.50 ≤56.00岁 63 5.00 ≤2.85 g/L 63 10.00 肝硬化(例) 0.05 0.816 ALP(例) 4.08 0.043 有 96 6.00 >106.00 U/L 54 4.50 无 13 6.00 ≤106.00 U/L 57 10.00 肿瘤数目(例) 0.39 0.532 GGT(例) 10.20 0.001 >1个 90 5.00 >73.50 U/L 55 4.00 1个 21 5.00 ≤73.50 U/L 56 12.00 肿瘤大小(例) 2.21 0.137 NLR(例) 0.03 0.856 >5 cm 62 5.00 >2.44 55 6.00 ≤5 cm 43 8.00 ≤2.44 56 6.00 AFP(例) 10.04 0.002 LMR(例) 0.12 0.727 >67.65 ng/μl 54 4.00 >4.43 55 6.00 ≤67.65 ng/μl 57 14.00 ≤4.43 56 6.00 TBA(例) 0.27 0.601 RBC(例) 0.89 0.345 >12.20 μmol/L 43 6.00 >4.40×1012/L 49 5.00 ≤12.20 μmol/L 68 6.00 ≤4.40×1012/L 62 6.00 TBil(例) 1.73 0.188 CXCL13(例) 7.90 0.005 >14.35 μmol/L 55 6.00 >81.23 pg/ml 55 10.00 ≤14.35 μmol/L 56 6.00 ≤81.23 pg/ml 56 4.50 ALT(例) 0.91 0.339 PT(例) 2.28 0.131 >35.50 U/L 55 7.00 >12.90 s 50 8.00 ≤35.50 U/L 56 6.00 ≤12.90 s 61 5.00 AST(例) 3.66 0.056 INR(例) 1.41 0.235 >39.50 U/L 55 5.00 >1.09 45 5.00 ≤39.50 U/L 56 12.00 ≤1.09 66 6.00 PA(例) 0.00 0.962 PLT(例) 0.28 0.596 >160.50 mg/L 47 6.00 >147.00×109/L 52 6.00 ≤160.50 mg/L 64 6.00 ≤147.00×109/L 59 6.00 Alb(例) 1.04 0.307 Child分级(例) 0.66 0.719 >40.50 g/L 55 5.00 A级 93 6.00 ≤40.50 g/L 56 8.00 B级 5 3.00 Glo(例) 0.98 0.320 BCLC分期(例) 12.36 <0.001 >31.50 g/L 52 5.00 A 64 15.00 ≤31.50 g/L 59 7.00 B 20 6.00 C 22 4.00 表 3 与肝癌复发相关的单因素分析
指标 β HR (95%CI) P值 AFP(ng/μl) 0.734 2.08(1.28~3.39) 0.003 TBA(μmol/L) 0.123 1.13(0.70~1.83) 0.618 TBil(μmol/L) 0.302 1.35(0.84~2.17) 0.211 ALT(U/L) -0.220 0.80(0.50~1.29) 0.803 AST(U/L) 0.436 1.55(0.96~2.48) 0.070 PA(mg/L) -0.011 0.99(0.61~1.61) 0.964 Alb(g/L) -0.235 0.79(0.49~1.27) 0.332 Glo(g/L) 0.231 1.26(0.78~2.04) 0.347 A/G -0.404 0.67(0.42~1.08) 0.096 FIB(g/L) 0.530 1.17(1.05~2.74) 0.030 ALP(U/L) 0.461 1.59(0.99~2.54) 0.056 GGT(U/L) 0.749 2.12(1.29~3.47) 0.003 NLR 0.168 1.18(0.68~2.06) 0.550 LMR 0.100 1.10(0.68~1.78) 0.683 RBC(×1012/L) 0.216 1.24(0.78~1.99) 0.369 CXCL13(pg/ml) -0.666 0.51(0.31~0.84) 0.009 PT(s) 0.345 1.41(0.88~2.26) 0.152 INR 0.274 1.32(0.82~2.12) 0.259 PLT(×109/L) 0.123 1.13(0.70~1.82) 0.614 表 4 Cox模型预测不同时间段复发情况的AUC、灵敏度及特异度
时间 复发率 AUC(95%CI) 灵敏度 特异度 0~3个月 30.63% 0.82(0.74~0.89) 91.18% 63.64% 0~6个月 50.45% 0.68(0.59~0.77) 71.43% 63.64% 0~12个月 56.76% 0.65(0.55~0.74) 68.25% 64.58% 0~24个月 60.36% 0.61(0.51~0.70) 64.18% 61.36% 表 5 Cox模型预测姑息性切除和根治性切除不同时间段复发情况的AUC、灵敏度及特异度
方法 例数 时间 AUC(95%CI) 灵敏度 特异度 姑息性切除 72 0~3个月 0.87(0.77~0.94) 93.75% 70.00% 0~6个月 0.72(0.60~0.82) 72.92% 66.67% 0~12个月 0.68(0.56~0.79) 69.81% 68.42% 0~24个月 0.63(0.51~0.74) 92.86% 37.50% 根治性切除 39 0~3个月 0.50(0.34~0.66) 100.00% 32.43% 0~6个月 0.57(0.40~0.72) 62.50% 61.29% 0~12个月 0.54(0.31~0.70) 50.00% 72.41% 0~24个月 0.51(0.35~0.67) 0 82.14% -
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. [2] ZHOU Z, LIN X, LIN N, et al. Establishment and predictive value of clinical prediction model for the recurrence after resection of hepatocellular carcinoma[J/CD]. Chin J Hepat Surg(Electronic Edition), 2017, 6(3): 192-196. DOI: 10.3877/cma.j.issn.2095-3232.2017.03.010.周正, 林晓, 林楠, 等. 肝细胞癌切除术后复发的临床预测模型的建立及其预测价值[J/CD]. 中华肝脏外科手术学电子杂志, 2017, 6(3): 192-196. DOI: 10.3877/cma.j.issn.2095-3232.2017.03.010. [3] ZLOTNIK A, YOSHIE O. The chemokine superfamily revisited[J]. Immunity, 2012, 36(5): 705-716. DOI: 10.1016/j.immuni.2012.05.008. [4] LI B, SU H, CAO J, et al. CXCL13 rather than IL-31 is a potential indicator in patients with hepatocellular carcinoma[J]. Cytokine, 2017, 89: 91-97. DOI: 10.1016/j.cyto.2016.08.016. [5] DUAN Z, GAO J, ZHANG L, et al. Phenotype and function of CXCR5+CD45RA-CD4+ T cells were altered in HBV-related hepatocellular carcinoma and elevated serum CXCL13 predicted better prognosis[J]. Oncotarget, 2015, 6(42): 44239-44253. DOI: 10.18632/oncotarget.6235. [6] BINDEA G, MLECNIK B, TOSOLINI M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer[J]. Immunity, 2013, 39(4): 782-795. DOI: 10.1016/j.immuni.2013.10.003. [7] Ministry of Health of the People's Republic of China. Diagnosis, management, and treatment of hepatocellular carcinoma (V2011)[J]. J Clin Hepatol, 2011, 27(11): 1141-1159. DOI: 10.3969/j.issn.1001-5256.2011.11.004中华人民共和国卫生部. 原发性肝癌诊疗规范(2011年版)[J]. 临床肝胆病杂志, 2011, 27(11): 1141-1159. DOI: 10.3969/j.issn.1001-5256.2011.11.004 [8] LI CY. Expression and effect of CXCL 13 chemokine in hepatocellullar carcinoma[D]. Changchun: Jilin University, 2017.李春艳. 趋化因子CXCL13在肝细胞癌中的表达及作用机制研究[D]. 长春: 吉林大学, 2017. [9] LI C, KANG D, SUN X, et al. The effect of C-X-C motif chemokine 13 on hepatocellular carcinoma associates with Wnt signaling[J]. Biomed Res Int, 2015, 2015: 345413. DOI: 10.1155/2015/345413. [10] FÖRSTER R, MATTIS AE, KREMMER E, et al. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen[J]. Cell, 1996, 87(6): 1037-1047. DOI: 10.1016/s0092-8674(00)81798-5. [11] KIM CH, ROTT LS, CLARK-LEWIS I, et al. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells[J]. J Exp Med, 2001, 193(12): 1373-1381. DOI: 10.1084/jem.193.12.1373. [12] CUCCHETTI A, PISCAGLIA F, CATURELLI E, et al. Comparison of recurrence of hepatocellular carcinoma after resection in patients with cirrhosis to its occurrence in a surveilled cirrhotic population[J]. Ann Surg Oncol, 2009, 16(2): 413-422. DOI: 10.1245/s10434-008-0232-4. [13] CHEN K, YANG HJ, DENG XF, et al. Risk factors of primary hepatocellular carcinoma patients early term recurrence after radical resection and prediction model establishment[J]. Chin J Cancer Prev Treat, 2018, 25(5): 344-348. https://www.cnki.com.cn/Article/CJFDTOTAL-QLZL201805009.htm陈凯, 杨洪吉, 邓小凡, 等. 肝癌根治性切除术后早期复发危险因素分析及预测模型构建[J]. 中华肿瘤防治杂志, 2018, 25(5): 344-348. https://www.cnki.com.cn/Article/CJFDTOTAL-QLZL201805009.htm [14] PAN YX, CHEN JC, FANG AP, et al. A nomogram predicting the recurrence of hepatocellular carcinoma in patients after laparoscopic hepatectomy[J]. Cancer Commun (Lond), 2019, 39(1): 55. DOI: 10.1186/s40880-019-0404-6. [15] YE YJ, LIU B, CHEN W, et al. Prognosis and staging system analysis of hepatocellular carcinoma patients with negative serum alpha-fetoprotein[J]. J Clin Hepatol, 2019, 35(3): 535-541. DOI: 10.3969/j.issn.1001-5256.2019.03.017.叶颖剑, 刘波, 陈伟, 等. 血清AFP阴性的肝细胞癌患者预后及分期系统分析[J]. 临床肝胆病杂志, 2019, 35(3): 535-541. DOI: 10.3969/j.issn.1001-5256.2019.03.017. [16] LIU AX, WANG HQ, BO WT, et al. Clinical efficacy and prognostic factors analysis of hepatectomy for hepatocellular carcinoma[J]. Chin J Dig Surg, 2019, 18(4): 368-374. DOI: 10.3760/cma.j.issn.1673-9752.2019.04.012.刘爱祥, 王海清, 薄文滔, 等. 肝细胞癌肝切除术的临床疗效及预后因素分析[J]. 中华消化外科杂志, 2019, 18(4): 368-374. DOI: 10.3760/cma.j.issn.1673-9752.2019.04.012. [17] XUE Y, ZHANG J, BAI Y, et al. Establishment and validation of a prognostic model for hepatocellular carcinoma after radical liver resection[J]. J BUON, 2019, 24(4): 1420-1428. http://www.researchgate.net/publication/336824473_Establishment_and_validation_of_a_prognostic_model_for_hepatocellular_carcinoma_after_radical_liver_resection/download [18] Department of Medical Administration. National Health and Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001 -5256.2020.02.007.中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗规范(2019年版)[J]. 临床肝胆病杂志, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001 -5256.2020.02.007. 期刊类型引用(3)
1. 亢必勃,赵绒,赵杨,申焕君,陈朝霞,周云,范超. 血浆B细胞趋化因子1和干细胞因子水平与HBV感染患者临床指标的关系. 基础医学与临床. 2023(02): 289-292 .  百度学术
百度学术2. 付雪,宁登,陈劲,杜鹏程,刘秋梦,姜立. eEF1A1通过HGF/c-MET通路调控肝癌细胞的迁移和侵袭能力. 中国组织化学与细胞化学杂志. 2023(02): 119-127 .  百度学术
百度学术3. 赵晨晨,杨方,王伟. 干扰素联合肝动脉化疗栓塞和门静脉化疗对预防肝癌术后复发的临床意义分析. 中国现代药物应用. 2022(03): 150-152 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 2259 KB)
PDF下载 ( 2259 KB)

 下载:
下载:


 下载:
下载:
 百度学术
百度学术