体外HBV感染的人肝癌Huh7细胞系中葡萄糖神经酰胺合成酶活性的变化
DOI: 10.3969/j.issn.1001-5256.2021.04.021
Change in the activity of glucosylceramide synthase in human hepatoma cell line with hepatitis B virus infection in vitro
-
摘要:
目的 探索糖鞘脂代谢合成关键酶葡萄糖神经酰胺(GCS)在体外HBV感染Huh7细胞中的活性变化。 方法 收集2019年6月—8月就诊于兰州大学第一医院感染科的9例初治急性乙型肝炎患者的血液标本,另选7例健康体检者的血液标本作为对照。利用HBV感染者血清中高拷贝HBV颗粒(>9.9×107 IU/ml)接种Huh7细胞系(感染组),对照组用健康体检者的血清共培养。检测HBV感染细胞胞浆中HBsAg和HBV DNA的表达量以及GCS的活性变化与病毒的相关性。计量资料多组间数据比较采用单因素方差分析,进一步两两比较采用LSD-t检验。相关性分析采用Pearson检验。 结果 感染组细胞较同期对照组细胞数量明显减少、细胞体积肿胀和细胞膜破碎。感染组:感染4 h、8 h、2 d和5 d后胞浆中HBsAg表达量均高于感染前(P<0.05);与感染4 h(16.67±11.55) IU/ml相比,感染8 h(112.01±25.94) IU/ml、2 d(328.01±103.50) IU/ml和5 d(101.60±49.84) IU/ml后胞浆中HBV DNA表达量呈明显升高趋势(P值均<0.001),并在感染2 d时HBV DNA表达量达到最高。在HBV感染期间,随着病毒复制的增加,GCS活性呈现出增高的特点,从感染4 h(126.21±9.59) IU/ml开始逐渐升高,并于感染2 d时(226.53±36.27) IU/ml达到高峰,感染2 d时与对照组(136.50±15.44) IU/ml比较,差异有统计学意义(t=3.956,P=0.016 7)。GCS活性与HBV DNA水平存在显著正相关(r=0.576 8, P=0.047 1)。 结论 HBV感染者血清中高拷贝HBV颗粒能成功感染Huh7细胞系,并在一定程度上模拟了HBV感染的体外特点。GCS活性可能与HBV的感染有关,表明糖鞘脂合成代谢与HBV可能存在密切的联系。 -
关键词:
- 乙型肝炎病毒 /
- 葡萄糖神经酰胺合成酶 /
- 体外培养技术
Abstract:Objective To investigate the change in the activity of glucosylceramide synthase, the key enzyme in glycosphingolipid metabolism and synthesis, in Huh7 cells infected by hepatitis B virus (HBV) in vitro. Methods Blood samples were collected from nine previously untreated patients with acute hepatitis B who attended Department of Infectious Diseases, The First Hospital of Lanzhou University, from June to August, 2019, and the blood samples collected from seven healthy individuals who underwent physical examination were established as control. Huh7 cells were inoculated with the high-copy HBV particles (> 9.9×107 IU/ml) in the serum of patients with HBV infection (infection group), and Huh7 cells co-cultured with the serum of healthy individuals were established as control group. The expression levels of HBsAg and HBV DNA in the cytoplasm of HBV-infected Huh7 cells were measured, and the correlation between GCS activity and virus was analyzed. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups, and a Pearson correlation analysis was performed. Results Compared with the control group, the infection group had a significant reduction in the number of cells, an increase in cell volume, and cell membrane fragmentation. The infection group had a significant increase in the expression of HBsAg in cytoplasm at 4 hours, 8 hours, 2 days, and 5 days after infection (P < 0.05); the expression level of HBV DNA tended to increase significantly from 4 hours after infection to 8 hours, 2 days, and 5 days after infection (16.67±11.55 IU/ml vs 112.01±25.94 IU/ml/328.01±103.50 IU/ml/101.60±49.84 IU/ml, P < 0.001), with the highest level at 2 days after infection. During HBV infection, the activity of GCS gradually increased with the increase in viral replication from 4 hours after infection (126.21±9.59 IU/ml) and reached a peak at 2 days after infection (226.53±36.27 IU/ml), with a significant difference between the infection group and the control group at 2 days after infection (226.53±36.27 IU/ml vs 136.50±15.44 IU/ml, t=3.956, P=0.016 7). The activity of GCS was positively correlated with HBV DNA level (r=0.576 8, P=0.047 1). Conclusion Huh7 cells are successfully infected with the high-copy HBV particles in the serum of patients with HBV infection, which mimics the characteristics of HBV infection in vitro to a certain degree. The activity of GCS may be associated with HBV infection, suggesting that glycosphingolipid synthesis and metabolism may be closely associated with HBV. -
Key words:
- Hepatitis B virus /
- Glucosylceramide Synthetase /
- In Vitro Techniques
-
HBV是一种小包膜DNA病毒,属于噬肝病毒科。尽管目前有乙型肝炎疫苗,但慢性乙型肝炎仍然是一个全球面临的重大公共卫生问题[1-2]。慢性HBV感染后可能导致肝炎、肝硬化、肝细胞癌[3],严重危害人类健康。在研究HBV致病机制和开发新型抗HBV药物过程中均离不开高质量的HBV感染模型,但是,由于HBV感染条件需具备高度种属特异性和组织特异性,使得建立合适的HBV感染模型成为制约乙型肝炎发病机制研究的瓶颈[4]。目前,已有体外感染鸭肝炎病毒和土拨鼠肝炎病毒动物模型的相关研究[5-6],这为下一步深入研究HBV感染人类的相关机制开阔了思路。近年来,HBV体外感染模型也有一定的进展,并能构建出稳定表达HBV的细胞系[7]。人源化的肝脏衍生细胞与人类亲缘关系近,克服了HBV感染的种属特异性和组织特异性,是研究HBV感染、发病机制和开发药物的有效方法。
鞘脂是细胞膜结构的重要组成部分,是由鞘氨醇、脂肪酸和磷酸胆碱组成的两性脂类,鞘氨醇是所有鞘脂共同的基本结构骨架,其上连接脂肪酸衍生物则构成神经酰胺(ceramide,Cer),是鞘脂代谢的中心分子[8]。Cer进一步在头基位置上连接一个磷酸胆碱则构成鞘磷脂类,如果其头基位置连接的是聚糖分子则构成糖鞘脂类,如连接葡萄糖则构成葡萄糖神经酰胺(glucosylceramide,GC)。Cer到GC的转变由葡萄糖神经酰胺合成酶(glucosylceramide synthetase,GCS)催化,该酶是糖鞘脂代谢的关键酶[9]。GCS参与众多病理生理过程,在细胞增殖、凋亡、耐药等方面扮演重要角色[9-11]。同时,在众多癌症中亦观察到GCS过度表达,GCS可促进相关恶性肿瘤发生发展,这表明开发出一些GCS的抑制类药物可能是好的治疗靶点[12]。近来,GCS与肝脏疾病的相关研究日益受到关注,并为肝病的诊治提供了一种有效策略。然而,GCS与HBV所致病毒性肝炎的关系的研究极为少见,以及GCS活性在HBV感染过程中的变化仍不明确。本研究在能较好的模拟HBV感染的体外模型基础上进行研究,并探索糖鞘脂代谢关键酶GCS在HBV感染体外细胞系过程中的活性变化。
1. 材料与方法
1.1 材料收集
收集2019年6月—8月就诊于兰州大学第一医院感染科的9例初治急性乙型肝炎患者的血液标本,所有患者HBV DNA滴度>9.9×107IU/ml;另选7例健康体检者的血液标本作为对照,其HBV定量均为阴性。
1.2 试剂和仪器
碘海醇购自Axis-shield公司(挪威),Huh7细胞系购自普诺赛生命科技有限公司(武汉),胎牛血清、DMEM和青-链霉素双抗购自Biological Industries公司(以色列),超速离心管和Himac超速离心机购自日立公司(日本),矿物油购自sigma公司(美国),荧光定量PCR试剂盒购自天隆生物科技有限公司(苏州),引物合成(英潍捷基有限公司,上海),相差显微镜购自Olympus公司(日本),人GCS-Elisa试剂盒购自江莱生物有限公司(上海),荧光定量PCR仪、人HBsAg检测试剂盒Elecsys HBsAg Ⅱ quant Ⅱ、Cobas e 601全自动免疫分析仪-电化学发光法购自Roche公司(瑞士)。
1.3 HBV颗粒纯化
使用DMEM培养基溶解碘海醇,定容到10 ml,4 ℃,旋转过夜溶解,用孔径为0.22 μm滤膜过滤除菌。用无菌DMEM培养基稀释质量体积比为42%、33%、25%、16%和8%的碘海醇溶液,在超速离心管内从高浓度到低浓度组份分别依次加入1.42 ml碘海醇溶液,然后在上部载入1.5 ml含有HBV的患者血清,最后在最上部盖上预冷矿物油。在超速离心机上以35 000 r/min,4 ℃的条件离心5.2 h,设置加减速为升4降5。离心完毕后取出离心管,弃掉表面矿物油,从上到下先取1 ml组份4个,然后取400 μl的组份分离离心以收集病毒。以上病毒实验的操作在二级安全实验室生物安全柜内严格按照相关规定操作。
1.4 细胞培养
人肝癌细胞系(Huh7)在含有10%胎牛血清、2 mmol/L的L-谷氨酰胺、4.5 g/L葡萄糖、2%DMSO、100 U/ml青霉素和100 μg/ml链霉素的DMEM培养基中培养。在培养瓶中接种约106个细胞,每1~2 d更换培养基1次。将从HBV阳性血清超速离心纯化所得病毒通过real-time qPCR的方法进行检测,选择病毒含量最高的4个组分进行混合,用于感染细胞。
1.5 HBV感染细胞
在接种后2~6 d,感染组用患者血清的1 ml HBV感染颗粒感染Huh7细胞系,对照组用来自健康体检者的血清共培养。在37 ℃温育3 h后,除去接种物后用PBS彻底洗涤细胞,并向细胞中加入补充有10-4 mol/L地塞米松和10-5 mol/L胰岛素的完全培养基。将培养物温育1 d,洗涤3次,并再次用新鲜培养基培养,孵育1 d。然后将细胞保持在补充有10-6 mol/L地塞米松和10-6 mol/L胰岛素的完全培养基中,每2 d更换培养基1次。待培养结束后收集细胞,采用反复冻融法裂解细胞,收获细胞胞浆,分别在4 h、8 h、2 d和5 d检测HBV抗原和HBV DNA。
1.6 细胞胞浆DNA测定
通过使用荧光定量PCR仪进行HBV DNA定量。用于实时PCR反应的引物和探针序列为:正向引物,5′-CCGTCTGTGCCTTCTCATCTG-3′;反向引物,5′-AGTCCAAGAGTYCTCTTATGYAAGACCTT-3′;探针,5′-FAM-CCGTGTGCACTTCGCTTCACCTCTGC-TAMRA-3′。扩增设置为0.8 μmol/L引物和0.2 μmol/L探针,总体系为20 μl。扩增条件为95 ℃预变性3 min,然后以94 ℃、15 s,60 ℃、30 s扩增45个循环。
1.7 HBsAg检测
将上述分离出的HBV感染的Huh7细胞胞浆和培养液上清采用电化学发光法全自动分析仪进行检测,具体操作步骤严格按照仪器说明书实行,简单来说:(1)将冷藏的人HBsAg平衡至室温(20 ℃~25 ℃),放置于分析仪的试剂盘内,并将试剂盒附带的定标液放置于分析仪的样本区,随后分析仪自动完成定标过程;(2)定标完成后,弃去定标液,在分析仪各对应样本区放置待测样本,分析仪会对样品进行1∶ 400稀释并读取结果。以上过程由分析仪自动进行。
1.8 GCS活性检测
将上述分离出的HBV感染的Huh7细胞胞浆和培养液上清采用ELISA法检测GCS活性,具体操作步骤严格按照仪器说明书实行。
1.9 伦理学审查
本研究方案经由兰州大学第一医院伦理委员会的批准,批号:LDYYLL2018-100,患者均签署知情同意书。
1.10 统计学方法
采用Graphpad 6.0软件进行统计分析。计量资料以x±s表示,多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。采用Pearson相关性分析GCS活性与HBV DNA的相关关系。P<0.05为差异有统计学意义。
2. 结果
2.1 细胞感染前后变化
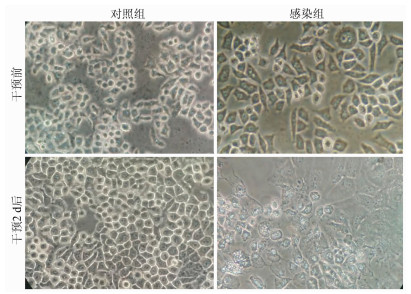
正常培养的细胞呈多边形或类圆形、有立体感,边界清晰,细胞核位于正中。对照组细胞符合上述特点,培养2 d后细胞明显增多。干预前的感染组细胞同样符合正常培养的细胞特点,感染HBV并培养2 d后细胞数量较感染前和对照组明显减少,其细胞体积肿胀,细胞膜破碎,表面出现胞膜疱,胞质颗粒粗糙,部分细胞脱壁,由于大部分细胞破碎脱落后可见核已消失的细胞胞浆或残屑(图 1)。
2.2 不同时间点感染组细胞中HBsAg和HBV DNA的表达量
感染4 h、8 h、2 d和5 d后胞浆中HBsAg表达量均高于感染前(P值均<0.05),表明HBV成功感染Huh7细胞系;与感染4 h相比,感染8 h、2 d和5 d后胞浆中HBV DNA表达量明显升高(P值均<0.001),并在感染2 d后HBV DNA达到最高(表 1)。
表 1 各时间点HBV感染细胞胞浆中HBsAg和HBV DNA的表达量时间 HBsAg(COI) HBV DNA(IU/ml) 感染前 <1 0 感染4 h 19.99±2.14 16.67±11.55 感染8 h 25.31±7.06 112.01±25.94 感染2 d 43.35±16.63 328.01±103.50 感染5 d 17.20±6.64 101.60±49.84 F值 9.60 18.32 P值 0.001 9 <0.001 2.3 GCS在HBV感染细胞中的活性变化及和病毒的相关性
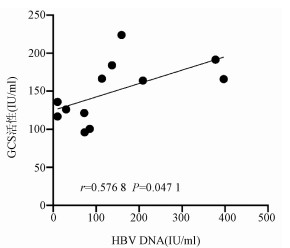
在HBV感染期间,随着病毒复制的增加,GCS活性呈现出增高的特点,从HBV感染4 h开始逐渐升高,并于感染2 d达到高峰,随后表现出下降趋势(表 2)。感染2 d与对照组相比,差异有统计学意义(t=3.956,P=0.016 7)。Pearson相关性分析显示,GCS活性与HBV DNA存在显著正相关关系(r=0.576 8, P=0.047 1)(图 2)。
表 2 GCS在HBV感染期间的活性变化分组 GCS(IU/ml) 对照组 136.50±15.44 感染组 感染4 h 126.21±9.59 感染8 h 167.40±23.62 感染2 d 226.53±36.27 感染5 d 108.60±17.83 3. 讨论
原代肝细胞是HBV感染的理想细胞,但由于其不能连续传代限制了其在研究中的使用。近期有学者发现,在将病毒接种到细胞之前去除细胞表面结合的脂蛋白有利于HBV感染肝细胞的入胞过程和后续HBV DNA的复制[13];也有研究[14]表明,Na+牛黄胆酸协同转运多肽为功能性HBV受体,通过对常用的肝细胞衍生细胞(即HepG2和Huh7)中重建表达Na+牛黄胆酸协同转运多肽后,细胞对HBV感染效率明显增强,促进了HBV生命周期早期感染机制和治疗的研究。本研究正是利用肝脏细胞衍生细胞Huh7,采用乙型肝炎患者HBV DNA高拷贝血清通过进一步纯化后进行感染。结果显示,在感染初期DNA复制低,这可能是HBV刚进入细胞后,进行复制、繁殖,生成成熟的病毒颗粒需要一段时间,也可能与外源DNA纯度有关。经过一段时间的培养后(即从8 h开始)病毒在细胞内进行持续复制并高表达,以上初步确定HBV DNA可以进入Huh7并进行复制。实验过程中添加了适量的胰岛素,其可以通过激活细胞转录因子调节HBV X蛋白的表达,将HBV基因组整合到细胞DNA中,以促进HBV进入细胞并进行复制[15];同时还通过梯度离心对HBV颗粒进行提纯,也增加了HBV感染细胞的概率。
研究[16-17]表明鞘脂家族成员在病毒性肝炎中扮演重要角色,如机体对抗病毒治疗的反应会伴随血清鞘脂水平变化,鞘脂在慢性病毒性肝炎感染中被认为是有希望的新生物标志物。但目前关于糖鞘脂代谢的关键酶GCS与病毒性肝炎的直接研究鲜有报道。由于HBV等哺乳动物病毒并没有独立编码自己的碳水化合物修饰的酶系统,只能利用宿主细胞糖基化机制来修改其病毒糖蛋白,如病毒糖蛋白的折叠[18],以达到侵入细胞并进行感染的目的,因此糖基化酶可能是病毒进行侵入和感染的重要物质。如果给予相应的糖基化酶抑制剂则可能干扰病毒糖蛋白的折叠,发挥抗病毒感染的作用。虽然目前并无GCS抑制剂直接用于抗病毒的证据,但GCS抑制剂用于其他疾病特别是癌症相关研究已得到充分证明[12, 19],并且其在抑制细菌生长方面也有显著成效[20]。糖鞘脂存在于所有哺乳动物细胞膜上,细胞膜是外界微生物入侵细胞的门户,因此糖鞘脂常被细菌和病毒用作受体并不稀奇。在糖鞘脂结构中,其脂性部分常常镶嵌入质膜中,而糖链部分则留在细胞外。糖基化会极大程度的改变原本脂质的功能特性,如Cer可通过诱导细胞周期阻滞和细胞凋亡发挥强大的抗癌作用,而其糖基化后的产物则产生截然相反的作用[9]。GCS作为糖鞘脂代谢的首部关键酶,其活性决定着细胞内Cer/GC的平衡,并影响细胞功能状态,进而影响HBV感染细胞成功与否。本研究发现在HBV感染Huh7期间,随着病毒复制的增加,GCS活性逐渐升高,这与上述观点相符合,因为病毒持续复制并感染越来越多的细胞,可能需要借助宿主细胞GCS修饰其病毒糖蛋白,协助病毒入侵细胞膜和转运的过程,当然这一过程也不能排除其糖基化产物在其中所起的作用。在之后,GCS的活性随着HBV DNA复制的减少而降低,以上初步表明GCS活性可能与HBV的感染有关,糖鞘脂代谢与乙型肝炎有密切的联系。
理想的HBV感染模型应该具备HBV感染率高、感染维持的时间长,且感染后能发生相应病理改变的优点,最好同时具有正常免疫系统,以便于评价免疫反应。虽然HBV感染的实验动物模型研究与应用已取得了较大进展,但除灵长类动物外的其他动物与人类亲缘关系较远,在模拟HBV感染人的致病机制和相应的病理生理改变方面存在较大限制。由于人体肝组织样本和灵长类动物模型来源十分有限,难以满足庞大的实验需求,所以人源化的衍生细胞系则具有重要的潜在应用价值。该细胞与人类亲缘关系近,易于被HBV感染,能极好的再现HBV感染人体的变化过程,但其也存在明显的缺点,由于体外细胞不具备体内完整的免疫系统,并且该细胞仍未达到理想的感染效率,因此不能观察到相应的免疫反应。
另外,本研究只是对GCS活性与HBV感染关系的初步研究,未能深入到具体机制。糖鞘脂家族成员众多,涉及的化学反应非常复杂,彼此间联系紧密。GCS作为糖鞘脂代谢的关键控制点,其活性直接决定着Cer/GC的平衡,从而对细胞命运起决定性作用,并能在一定程度上影响相关肝脏疾病的结局,如病毒性肝炎和肝细胞癌等。因此,有必要进一步研究GCS,在将来,GCS可能成为相关肝脏疾病的治疗靶点。
总之,本研究通过尝试在体外模拟HBV感染的特点,在此基础上探讨了糖鞘脂合成关键酶和HBV复制的可能关系,研究提示糖鞘脂的合成可能与HBV的复制有关,两者之间的密切关系有可能是HBV感染的新的关注点。
-
表 1 各时间点HBV感染细胞胞浆中HBsAg和HBV DNA的表达量
时间 HBsAg(COI) HBV DNA(IU/ml) 感染前 <1 0 感染4 h 19.99±2.14 16.67±11.55 感染8 h 25.31±7.06 112.01±25.94 感染2 d 43.35±16.63 328.01±103.50 感染5 d 17.20±6.64 101.60±49.84 F值 9.60 18.32 P值 0.001 9 <0.001 表 2 GCS在HBV感染期间的活性变化
分组 GCS(IU/ml) 对照组 136.50±15.44 感染组 感染4 h 126.21±9.59 感染8 h 167.40±23.62 感染2 d 226.53±36.27 感染5 d 108.60±17.83 -
[1] OMATA M, KANDA T, WEI L, et al. APASL consensus statements and recommendation on treatment of hepatitis C[J]. Hepatol Int, 2016, 10(5): 702-726. DOI: 10.1007/s12072-016-9717-6. [2] XU ZH, LIU Y, XU DP. Advances in novel anti-HBV agents acting on different targets[J]. Infect Dis Info, 2015, 28(5): 257- 261, 283. DOI: 10.3969/j.issn.1007-8134.2015.05.001.许智慧, 刘妍, 徐东平. 针对不同靶点的抗HBV新药研究进展[J]. 传染病信息, 2015, 28(5): 257-261, 283. DOI: 10.3969/j.issn.1007-8134.2015.05.001. [3] de MARTEL C, GEORGES D, BRAY F, et al. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis[J]. Lancet Glob Health, 2020, 8(2): e180-e190. DOI: 10.1016/S2214-109X(19)30488-7. [4] SU Y, ZHU YF, TIAN QY, et al. In vitro cell model and mouse model of HBV cccDNA[J]. J Clin Hepatol, 2019, 35(6): 1205- 1211. DOI: 10.3969/j.issn.1001-5256.2019.06.007.苏瑜, 朱园飞, 田青右, 等. HBV cccDNA的体外细胞模型和实验小鼠模型[J]. 临床肝胆病杂志, 2019, 35(6): 1205-1211. DOI: 10.3969/j.issn.1001-5256.2019.06.007. [5] NOORDEEN F, SCOUGALL CA, GROSSE A, et al. Therapeutic antiviral effect of the nucleic acid polymer REP 2055 against persistent duck hepatitis B virus infection[J]. PLoS One, 2015, 10(11): e0140909. DOI: 10.1371/journal.pone.0140909. [6] FLETCHER SP, CHIN DJ, GRUENBAUM L, et al. Intrahepatic transcriptional signature associated with response to interferon-α treatment in the woodchuck model of chronic hepatitis B[J]. PLoS Pathog, 2015, 11(9): e1005103. DOI: 10.1371/journal.ppat.1005103. [7] NI Y, LEMPP FA, MEHRLE S, et al. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes[J]. Gastroenterology, 2014, 146(4): 1070-1083. DOI: 10.1053/j.gastro.2013.12.024. [8] HANNUN YA, OBEID LM. Sphingolipids and their metabolism in physiology and disease[J]. Nat Rev Mol Cell Biol, 2018, 19(3): 175-191. DOI: 10.1038/nrm.2017.107. [9] LIU YY, HILL RA, LI YT. Ceramide glycosylation catalyzed by glucosylceramide synthase and cancer drug resistance[J]. Adv Cancer Res, 2013, 117: 59-89. DOI: 10.1016/B978-0-12-394274-6.00003-0. [10] LI JF, ZHENG SJ, LIU S, et al. Impact of UGCG siRNA on 7702 hepatocyte proliferation in vitro[J]. J Pract Hepatol, 2018, 21(5): 697-700. DOI: 10.3969/j.issn.1672-5069.2018.05.011.李俊峰, 郑素军, 刘霜, 等. 葡萄糖神经酰胺葡萄糖基转移酶siRNA对7702肝细胞增殖的影响及机制研究[J]. 实用肝脏病杂志, 2018, 21(5): 697-700. DOI: 10.3969/j.issn.1672-5069.2018.05.011. [11] WEGNER MS, GRUBER L, MATTJUS P, et al. The UDP-glucose ceramide glycosyltransferase (UGCG) and the link to multidrug resistance protein 1 (MDR1)[J]. BMC Cancer, 2018, 18(1): 153. DOI: 10.1186/s12885-018-4084-4. [12] WANG T, WEI J, WANG N, et al. The glucosylceramide synthase inhibitor PDMP sensitizes pancreatic cancer cells to MEK/ERK inhibitor AZD-6244[J]. Biochem Biophys Res Commun, 2015, 456(3): 821-826. DOI: 10.1016/j.bbrc.2014.12.019. [13] FAVRE D, PETIT MA, TRÉPO C. Latent hepatitis B virus (HBV) infection and HBV DNA integration is associated with further transformation of hepatoma cells in vitro[J]. ALTEX, 2003, 20(3): 131-142. [14] ZHONG G, YAN H, WANG H, et al. Sodium taurocholate cotransporting polypeptide mediates woolly monkey hepatitis B virus infection of Tupaia hepatocytes[J]. J Virol, 2013, 87(12): 7176-7184. DOI: 10.1128/JVI.03533-12. [15] CHOI BH, PARK CJ, RHO HM. Insulin activates the hepatitis B virus X gene through the activating protein-1 binding site in HepG2 cells[J]. DNA Cell Biol, 1998, 17(11): 951-956. DOI: 10.1089/dna.1998.17.951. [16] ZHENG SJ, QU F, LI JF, et al. Serum sphingomyelin has potential to reflect hepatic injury in chronic hepatitis B virus infection[J]. Int J Infect Dis, 2015, 33: 149-155. DOI: 10.1016/j.ijid.2015.01.020. [17] ZHANG JY, QU F, LI JF, et al. Up-regulation of plasma hexosylceramide (d18: 1/18: 1) contributes to genotype 2 virus replication in chronic hepatitis C: A 20-year cohort study[J]. Medicine (Baltimore), 2016, 95(23): e3773. DOI: 10.1097/MD.0000000000003773. [18] WERR M, PRANGE R. Role for calnexin and N-linked glycosylation in the assembly and secretion of hepatitis B virus middle envelope protein particles[J]. J Virol, 1998, 72(1): 778-782. DOI: 10.1128/JVI.72.1.778-782.1998. [19] XU J, ZHAO W, SUN J, et al. Novel glucosylceramide synthase inhibitor based prodrug copolymer micelles for delivery of anticancer agents[J]. J Control Release, 2018, 288: 212-226. DOI: 10.1016/j.jconrel.2018.09.011. [20] LEVERY SB, MOMANY M, LINDSEY R, et al. Disruption of the glucosylceramide biosynthetic pathway in Aspergillus nidulans and Aspergillus fumigatus by inhibitors of UDP-Glc: Ceramide glucosyltransferase strongly affects spore germination, cell cycle, and hyphal growth[J]. FEBS Lett, 2002, 525(1-3): 59-64. DOI: 10.1016/s0014-5793(02)03067-3. 期刊类型引用(0)
其他类型引用(1)
-




 PDF下载 ( 2434 KB)
PDF下载 ( 2434 KB)


 下载:
下载:

 下载:
下载: