胆汁中趋化因子C-X3-C-基元受体1和可溶性CD40配体在肝移植术后肝损伤中的预测价值
DOI: 10.3969/j.issn.1001-5256.2021.04.023
Value of Fractalkine and soluble CD40 ligand in bile in predicting liver injury after liver transplantation
-
摘要:
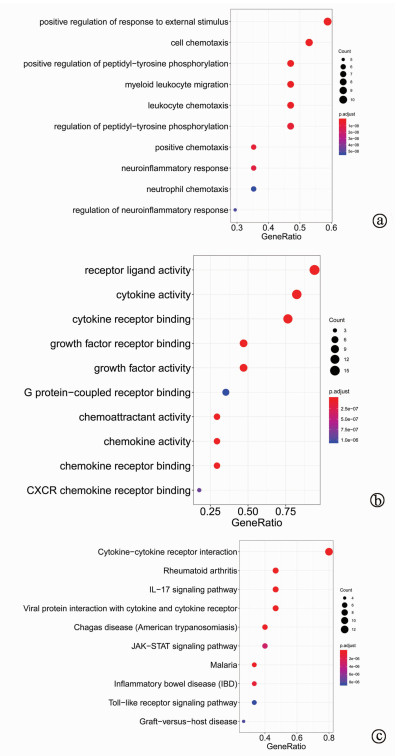
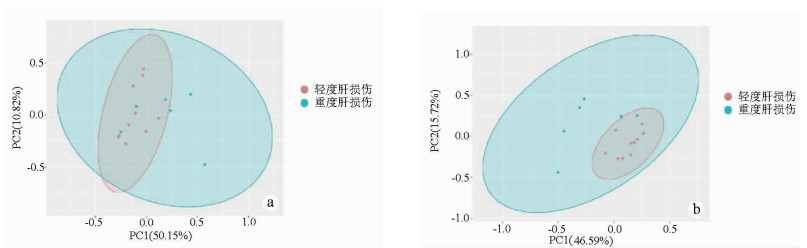
目的 探究胆汁中细胞因子联合临床指标对肝移植术后肝损伤程度的预测作用。 方法 选取2018年1月—12月青岛大学附属医院器官移植中心收治的16例肝移植患者。按术后第1天ALT水平分为轻度肝损伤(ALT<500 U/L,10例)和重度肝损伤(ALT>500 U/L,6例)。采集两组患者术后第1、3、5、7天的胆汁,应用MILLIPLEX®高通量多因子检测技术测定17种细胞因子水平。运用R软件,对胆汁中细胞因子和临床指标进行主成分分析(PCA),并对胆汁中细胞因子进行GO富集分析。符合正态分布的计量资料两组间比较采用两独立样本t检验;非正态分布的计量资料两组间比较采用Mann-Whitney U检验。采用Spearman相关性分析对临床指标与胆汁中细胞因子的相关性进行分析。采用受试者工作特征曲线(ROC曲线)分析评估胆汁中细胞因子及临床相关指标对肝移植术后肝损伤的预测价值。 结果 与轻度肝损伤组相比,重度肝损伤组胆汁中趋化因子C-X3-C-基元受体1 (Fractalkine)(Z=-2.828,P=0.003)、可溶性CD40配体(sCD40L)(Z=-2.850,P=0.008)、IL-4(Z=-2.398,P=0.017)、趋化因子CXCL10(Z=-2.475,P=0.023)和巨细胞炎性蛋白-1α(Z=-1.844,P=0.043)表达水平更高,差异均有统计学意义。相关性分析结果显示,肝移植术后第1天,AST、ALT和LDH与胆汁中多个细胞因子呈正相关(P值均<0.05)。Fractalkine、sCD40L、AST的ROC曲线下面积分别为0.933(0.812~1.000)、0.833(0.589~1.000)、0.917(0.779~1.000),提示术后第1天AST及胆汁中Fractalkine和sCD40L水平对肝移植术后肝损伤程度有明显预测价值。PCA分析结果显示,肝移植术后第1天胆汁中细胞因子结合临床指标可以将肝移植术后轻度与重度肝损伤患者较好地进行区分。GO分析结果显示,胆汁中细胞因子与外部刺激的正反馈调节、细胞趋化性、受体配体活性、细胞因子活性、细胞因子-细胞因子受体相互作用有关。 结论 胆汁中Fractalkine和sCD40L对肝移植术后肝损伤程度具有潜在的预测价值。 Abstract:Objective To investigate the value of cytokines in bile combined with clinical indices in predicting the degree of liver injury after liver transplantation. Methods A total of 16 patients undergoing liver transplantation who were hospitalized in Center of Organ Transplantation, The Affiliated Hospital of Qingdao University, from January to December 2018 were enrolled, and according to the level of alanine aminotransferase (ALT) on day 1 after surgery, the patients were divided into mild liver injury (ALT < 500 U/L) group with 10 patients and severe liver injury (ALT > 500 U/L) group with 6 patients. Bile samples were collected on days 1, 3, 5, and 7 after surgery, and MILLIPLEX® assay was used to measure the levels of 17 cytokines. R software was used to perform principal component analysis (PCA) of bile cytokines and clinical indices and gene ontology (GO) enrichment analysis of bile cytokines. The two-independent-samples t-test was used for comparison of normally distributed continuous data between two groups; The Mann-Whitney U test was used for comparison of non- normally distributed continuous data between two groups. A Spearman correlation analysis was performed to evaluate the correlation between clinical indices and bile cytokines. ROC curve analysis was used to evaluate the predictive value of cytokines in bile and clinical indices for liver injury after liver transplantation. Results Compared with the mild liver injury group, the severe liver injury group had significantly higher expression levels of bile Fractalkine (Z=-2.828, P=0.003), soluble CD40 ligand (sCD40L) (Z=-2.850, P=0.008), interleukin-4 (Z=-2.398, P=0.017), CXCL10 (Z=-2.475, P=0.023), and macrophage inflammatory protein-1α (Z=-1.844, P=0.043). The correlation analysis showed that on day 1 after liver transplantation, aspartate aminotransferase, ALT, and lactate dehydrogenase were positively correlated with the levels of several cytokines in bile (all P < 0.05). The area under the ROC curve of Fractalkine, sCD40L and AST were 0.933 (0.812-1.000), 0.833 (0.589-1.000) and 0.917 (0.779-1.000), respectively, suggesting that AST and Fractalkine and sCD40L in bile on the first day after liver transplantation have significant predictive value for liver injury. The results of PCA showed that bile cytokines combined with clinical indices on day 1 after liver transplantation could better distinguish the patients with mild liver injury from those with severe liver injury. GO analysis showed that bile cytokines were associated with positive feedback regulation of external stimulus, cell chemotaxis, receptor ligand activity, cytokine activity, and cytokine-cytokine receptor interaction. Conclusion Fractalkine and sCD40L in bile can predict the degree of liver injury after liver transplantation. -
Key words:
- Liver Transplantation /
- Cytokines /
- Hepatic Insufficiency
-
表 1 不同时间点两组患者ALT、AST、LDH水平变化
肝移植术后时间 指标 轻度肝损伤组(n=10) 重度肝损伤组(n=6) 统计值 P值 第1天 ALT(U/L) 352.0(179.3~478.8) 814.5(641.0~1159.0) Z=-3.254 <0.001 AST(U/L) 364.5 (207.5~494.5) 1039.0(751.5~2314.0) Z=-2.712 0.005 LDH(U/L) 601.0(334.0~931.3) 1719.0(1186.0~2066.0) Z=-2.820 0.003 第3天 ALT(U/L) 188.5(108.5~230.8) 422.0(373.3~906.5) Z=-3.254 <0.001 AST(U/L) 79.0(54.3~148.5) 236.5(160.8~625.3) Z=-2.495 0.011 LDH(U/L) 310.1(209.0~341.8) 345.5(218.3~476.3) Z=-2.820 0.410 第5天 ALT(U/L) 98.5(54.5~151.5) 214.5(175.0~500.5) Z=-3.037 0.001 AST(U/L) 31.5(27.5~52.0) 48.0(31.0~127.8) Z=-0.978 0.184 LDH(U/L) 267.40±80.53 275.00±104.40 t=0.164 0.872 第7天 ALT(U/L) 89.0(61.8~164.8) 95.0(64.3~193.8) Z=-0.434 0.595 AST(U/L) 31.5(19.8~88.3) 25.5(13.3~30.3) Z=-1.193 0.162 LDH(U/L) 272.00±78.01 196.00±50.20 t=2.122 0.052 表 2 轻度肝损伤与重度肝损伤胆汁中细胞因子比较
细胞因子 轻度肝损伤组(n=10) 重度肝损伤组(n=6) Z值 P值 Fractalkine(pg/ml) 64.180(0.000~108.700) 208.700 (146.600~281.400) -2.828 0.003 sCD40L(pg/ml) 0.000 (0.000~0.000) 5.665 (0.000~19.590) -2.850 0.008 IL-4(pg/ml) 1.885 (0.000~5.765) 15.890 (7.958~21.900) -2.398 0.017 IP-10(pg/ml) 951.500 (652.100~1374.000) 1637.000(1302.000~3113.000) -2.475 0.023 MIP-1α(pg/ml) 9.075(3.165~10.480) 11.030(9.450~18.200) -1.844 0.043 MDC(pg/ml) 2.655 (0.000~11.070) 21.590(3.878~27.790) -1.970 0.051 FGF-2(pg/ml) 35.440 (0.000~68.510) 84.600(47.200~159.700) -1.908 0.059 IL-1β(pg/ml) 0.350 (0.000~0.803) 1.295(0.555~2.860) -1.874 0.064 IL-2(pg/ml) 0.930 (0.793~1.148) 1.640(0.838~2.475) -1.684 0.099 IL-17A(pg/ml) 2.930(2.420~4.205) 5.225(2.485~6.313) -1.249 0.168 IFNγ(pg/ml) 0.355 (0.000~0.913) 1.965 (0.000~4.428) -1.360 0.190 VEGFα(pg/ml) 83.240 (32.290~134.100) 160.800(91.630~209.700) -1.955 0.052 G-CSF(pg/ml) 2.395 (0.000~9.593) 6.720 (1.598~23.560) -1.159 0.264 IL-15(pg/ml) 2.035 (0.548~2.393) 2.130 (0.735~3.633) -0.599 0.578 IL-8(pg/ml) 152.300(47.240~274.600) 150.900(118.500~207.400) -0.217 0.690 IL-6(pg/ml) 29.590 (1.298~71.360) 42.390(23.300~54.660) -0.271 0.814 TGFα(pg/ml) 13.580(6.863~22.870) 16.870(7.235~22.330) -0.325 0.879 注:FGF-2,纤维细胞生长因子-2;VEGFα,血管内皮生长因子α;G-CSF,粒细胞集落刺激因子。 表 3 临床指标与胆汁中细胞因子的相关性
指标 细胞因子 r值 P值 ALT AST 0.821 <0.001 LDH 0.782 <0.001 Fractalkine 0.569 0.021 sCD40L 0.653 0.006 MDC 0.614 0.011 FGF-2 0.520 0.039 IL-1β 0.644 0.007 VEGFα 0.538 0.035 AST LDH 0.932 <0.001 sCD40L 0.580 0.019 MDC 0.555 0.026 LDH sCD40L 0.576 0.020 IP-10 0.603 0.013 MDC 0.579 0.019 -
[1] LEE HW, SUH KS. Liver transplantation for advanced hepatocellular carcinoma[J]. Clin Mol Hepatol, 2016, 22(3): 309-318. DOI: 10.3350/cmh.2016.0042 [2] LIU H, LO CM, YEUNG O, et al. NLRP3 inflammasome induced liver graft injury through activation of telomere-independent RAP1/KC axis[J]. J Pathol, 2017, 242(3): 284-296. DOI: 10.1002/path.4901 [3] ZHAI Y, BUSUTTIL RW, KUPIEC-WEGLINSKI JW. Liver ischemia and reperfusion injury: New insights into mechanisms of innate-adaptive immune-mediated tissue inflammation[J]. Am J Transplant, 2011, 11(8): 1563-1569. DOI: 10.1111/j.1600-6143.2011.03579.x [4] HONG BJ, LIU H, WANG ZH, et al. Inflammasome activation involved in early inflammation reaction after liver transplantation[J]. Immunol Lett, 2017, 190: 265-271. DOI: 10.1016/j.imlet.2017.08.020 [5] CHALIN A, LEFEVRE B, DEVISME C, et al. Serum CXCL10, CXCL11, CXCL12, and CXCL14 chemokine patterns in patients with acute liver injury[J]. Cytokine, 2018, 111: 500-504. DOI: 10.1016/j.cyto.2018.05.029 [6] SAHIN H, BORKHAM-KAMPHORST E, DO O NT, et al. Proapoptotic effects of the chemokine, CXCL 10 are mediated by the noncognate receptor TLR4 in hepatocytes[J]. Hepatology, 2013, 57(2): 797-805. DOI: 10.1002/hep.26069 [7] JIMÉNEZ-CASTRO MB, CORNIDE-PETRONIO ME, GRACIA-SANCHO J, et al. Inflammasome-mediated inflammation in liver ischemia-reperfusion injury[J]. Cells, 2019, 8(10): 1131. DOI: 10.3390/cells8101131 [8] BARBIER L, FERHAT M, SALAMÉ E, et al. Interleukin-1 family cytokines: Keystones in liver inflammatory diseases[J]. Front Immunol, 2019, 10: 2014. DOI: 10.3389/fimmu.2019.02014 [9] LEA JD, CLARKE JI, MCGUIRE N, et al. Redox-dependent hmgb1 isoforms as pivotal co-ordinators of drug-induced liver injury: Mechanistic biomarkers and therapeutic targets[J]. Antioxid Redox Signal, 2016, 24(12): 652-665. DOI: 10.1089/ars.2015.6406 [10] FAN X, DU J, WANG MH, et al. Irisin contributes to the hepatoprotection of dexmedetomidine during intestinal ischemia/reperfusion[J]. Oxid Med Cell Longev, 2019, 2019: 7857082. http://www.ncbi.nlm.nih.gov/pubmed/31191804 [11] LADUE JS, WROBLEWSKI F. Serum glutamic pyruvic transaminase SGP-T in hepatic disease: A preliminary report[J]. Ann Intern Med, 1956, 45(5): 801-811. DOI: 10.7326/0003-4819-45-5-801 [12] KALTENBACH MG, HARHAY MO, ABT PL, et al. Trends in deceased donor liver enzymes prior to transplant: The impact on graft selection and outcomes[J]. Am J Transplant, 2020, 20(1): 213-219. DOI: 10.1111/ajt.15573 [13] MANGUS RS, FRIDELL JA, KUBAL CA, et al. Elevated alanine aminotransferase (ALT) in the deceased donor: Impact on early post-transplant liver allograft function[J]. Liver Int, 2015, 35(2): 524-531. DOI: 10.1111/liv.12508 [14] D'HAESE JG, DEMIR IE, FRIESS H, et al. Fractalkine/CX3CR1: Why a single chemokine-receptor duo bears a major and unique therapeutic potential[J]. Expert Opin Ther Targets, 2010, 14(2): 207-219. DOI: 10.1517/14728220903540265 [15] SUTTI S, HEYMANN F, BRUZZí S, et al. CX3CR1 modulates the anti-inflammatory activity of hepatic dendritic cells in response to acute liver injury[J]. Clin Sci (Lond), 2017, 131(17): 2289-2301. DOI: 10.1042/CS20171025 [16] EFSEN E, GRAPPONE C, DEFRANCO RM, et al. Up-regulated expression of fractalkine and its receptor CX3CR1 during liver injury in humans[J]. J Hepatol, 2002, 37(1): 39-47. DOI: 10.1016/S0168-8278(02)00065-X [17] OBARA H, NAGASAKI K, HSIEH CL, et al. IFN-gamma, produced by NK cells that infiltrate liver allografts early after transplantation, links the innate and adaptive immune responses[J]. Am J Transplant, 2005, 5(9): 2094-2103. DOI: 10.1111/j.1600-6143.2005.00995.x [18] SAFIRI S, ASHRAFI-ASGARABAD A. High serum soluble CD40L levels previously to liver transplantation in patients with hepatocellular carcinoma are associated with mortality at one year: Methodological issues[J]. J Crit Care, 2018, 43: 370. DOI: 10.1016/j.jcrc.2017.10.010 [19] ERCIN CN, DOGRU T, TAPAN S, et al. Levels of soluble CD40 ligand and P-Selectin in nonalcoholic fatty liver disease[J]. Dig Dis Sci, 2010, 55(4): 1128-1134. DOI: 10.1007/s10620-009-0817-1 [20] BERRES M, TRAUTWEIN C, SCHMEDING M, et al. Serum chemokine CXC ligand 10 (CXCL10) predicts fibrosis progression after liver transplantation for hepatitis C infection[J]. Hepatology, 2011, 53(2): 596-603. DOI: 10.1002/hep.24098 [21] ZIMMERMANN HW, TRAUTWEIN C, TACKE F. Functional role of monocytes and macrophages for the inflammatory response in acute liver injury[J]. Front Physiol, 2012, 3: 56. http://www.ncbi.nlm.nih.gov/pubmed/23091461 [22] BERKHOUT L, BARIKBIN R, SCHILLER B, et al. Deletion of tumour necrosis factor α receptor 1 elicits an increased TH17 immune response in the chronically inflamed liver[J]. Sci Rep, 2019, 9(1): 4232. DOI: 10.1038/s41598-019-40324-z [23] SUTTI S, BRUZZí S, HEYMANN F, et al. CX3CR1 mediates the development of monocyte-derived dendritic cells during hepatic inflammation[J]. Cells, 2019, 8(9): 1099. DOI: 10.3390/cells8091099 [24] DEAR J, CLARKE J, FRANCIS B, et al. Risk stratification after paracetamol overdose using mechanistic biomarkers: Results from two prospective cohort studies[J]. Lancet Gastroenterol Hepatol, 2018, 3(2): 104-113. DOI: 10.1016/S2468-1253(17)30266-2 -



 PDF下载 ( 2805 KB)
PDF下载 ( 2805 KB)

 下载:
下载: