STAT3在对乙酰氨基酚所致小鼠肝损伤后肝细胞再生中的作用
DOI: 10.3969/j.issn.1001-5256.2021.04.026
Role of STAT3 in hepatocyte regeneration after acetaminophen-induced hepatocellular injury in mice
-
摘要:
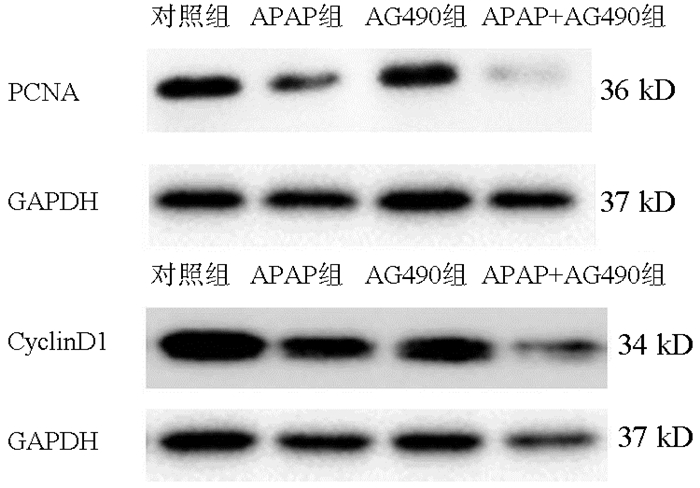
目的 探讨STAT3在对乙酰氨基酚(APAP)致小鼠肝细胞损伤后肝细胞增殖中的作用。 方法 体外培养小鼠正常肝细胞AML12,APAP(1、2.5、5、10、20 mmol/L)刺激12、24或48 h,等体积PBS作为对照组。筛选出最佳刺激浓度和作用时间后,AG490(10、50、100 μmol/L)作用AML12。CCK8法检测AML12细胞活力。RT-PCR检测AML12中PCNA、CyclinD1、Ki67的mRNA表达水平。Western Blot法检测STAT3、p-STAT3及PCNA、CyclinD1表达水平。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 APAP作用24 h及48 h后,与对照组比较,各浓度组AML12细胞活力均降低(P值均 < 0.05);APAP浓度为2.5 mmol/L时,细胞活力分别为0.717±0.027、0.752±0.014,与对照组比较差异均有统计学意义(P值均 < 0.05),能够满足后续实验条件。与对照组比较,24 h APAP(2.5 mmol/L)组PCNA、CyclinD1及Ki67 mRNA的表达均降低(P值均 < 0.01);与24 h APAP组比较,48 h APAP(2.5 mmol/L)组PCNA、CyclinD1及Ki67 mRNA的表达均升高(P < 0.01),因此选择APAP 2.5 mmol/L、刺激时间48 h来模拟体外损伤AML12细胞后肝细胞再生的模型。加入AG490,与对照组比较,10、50 μmol/L AG490组细胞活力变化无统计学意义,余各组细胞活力均降低(P值均 < 0.01);与APAP组比较,AG490(50 μmol/L)+APAP组和AG490(100 μmol/L)+APAP组细胞活力降低(P值均 < 0.01),因此选择50 μmol/L AG490作为后续实验处理浓度。与对照组比较,APAP组p-STAT3的蛋白水平升高(P < 0.01),而AG490组、APAP+AG490组降低(P值均 < 0.05);与APAP组比较,APAP+AG490组PCNA、CyclinD1蛋白水平及PCNA、CyclinD1、Ki67 mRNA表达均降低(P值均 < 0.05)。 结论 STAT3参与APAP诱导小鼠肝细胞损伤后的细胞增殖,而AG490作为STAT3抑制剂通过抑制STAT3磷酸化从而抑制APAP肝损伤后肝细胞增殖。 -
关键词:
- 化学性与药物性肝损伤 /
- STAT3转录因子 /
- 醋氨酚 /
- 肝再生
Abstract:Objective To investigate the role of STAT3 in hepatocyte proliferation after acetaminophen (APAP)-induced hepatocellular injury in mice. Methods Normal mouse AML12 hepatocytes were cultured in vitro and were stimulated by APAP (1, 2.5, 5, 10, and 20 mmol/L) for 12, 24 or 48 hours, and the hepatocytes treated with an equal volume of phosphate buffered saline were established as control group. After the optimal stimulation concentration and duration of action were screened out, AML12 hepatocytes were treated with AG490 (10, 50, and 100 μmol/L). The CCK-8 assay was used to measure the viability of AML12 hepatocytes; RT-PCR was used to measure the mRNA expression levels of PCNA, CyclinD1, and Ki67 in AML12 hepatocytes, and Western blot was used to measure the protein expression levels of STAT3, p-STAT3, PCNA, and CyclinD1. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results After 24 and 48 hours of APAP treatment, compared with the control group, all concentration groups had a significant reduction in the viability of AML12 hepatocytes (all P < 0.05), with a viability of 0.717±0.0271 and 0.752±0.0141, respectively, when the concentration of APAP was 2.5 mmol/L, which was significantly different from that in the control group (all P < 0.05) and met the conditions of subsequent experiment. Compared with the control group, the 24-hour APAP (2.5 mmol/L) group had significant reductions in the mRNA expression of PCNA, CyclinD1, and Ki67 (all P < 0.01); compared with the 24-hour APAP group, the 48-hour APAP (2.5 mmol/L) group had significant increases in the mRNA expression of PCNA, CyclinD1, and Ki67 (all P < 0.01); therefore, a model of hepatocyte regeneration after in vitro AML12 hepatocyte injury was established by stimulation with APAP 2.5 mmol/L for 48 hours. After the addition of AG490, there was no significant difference in viability between the control group and the 10 and 50 μmol/L AG490 groups, and the other groups had a significant reduction in viability (all P < 0.01); compared with the APAP group, the AG490 (50 μmol/L)+APAP group and the AG490 (100 μmol/L)+APAP group had a significant reduction in viability (P < 0.01); therefore, 50 μmol/L AG490 was selected as the concentration for subsequent experiment. Compared with the control group, the APAP group had a significant increase in the protein expression level of p-STAT3 (P < 0.01), while the AG490 group and the APAP+AG490 group had a significant reduction (both P < 0.05); compared with the APAP group, the APAP+AG490 group had significant reductions in the protein expression levels of PCNA and CyclinD1 and the mRNA expression levels of PCNA, CyclinD1, and Ki67 (all P < 0.05). Conclusion STAT3 participates in hepatocyte proliferation after APAP-induced hepatocyte injury in mice, while AG490, as an STAT3 inhibitor, can inhibit hepatocyte proliferation after APAP-induced hepatocyte injury by inhibiting the phosphorylation of STAT3. -
表 1 RT-PCR引物序列
引物名称 上游引物(5′-3′) 下游引物(5′-3′) 18S GTAACCCGTTGAACCCCATT CCATCCAATCGGTAGTAGCG PCNA TGAAGAAGGTGCTGGAGGCTCTC AGCTGTACCAAGGAGACGTGAGAC Ki67 GCCTGCCCGACCCTACAAAATG CTCATCTGCTGCTGCTTCTCCTTC CyclinD1 TGACTGCCGAGAAGTTGTGC CTCATCCGCCTCTGGCATT 表 2 CCK8检测APAP对AML12细胞活力的影响
组别 12 h 24 h 48 h 对照组 0.980±0.035 1.000±0.014 0.981±0.033 APAP 1 mmol/L组 1.010±0.042 0.931±0.0241) 0.911±0.0171) APAP 2.5 mmol/L组 0.971±0.029 0.717±0.0271) 0.752±0.0141) APAP 5.0 mmol/L组 0.925±0.040 0.672±0.0191) 0.331±0.0101) APAP 10.0 mmol/L组 0.918±0.063 0.596±0.0391) 0.224±0.0181) APAP 20.0 mmol/L组 0.982±0.019 0.601±0.0231) 0.095±0.0151) F值 2.306 137.023 1163.999 P值 0.087 < 0.001 < 0.001 注:与同一时间对照组比较,1)P<0.05。 表 3 APAP对AML12细胞增殖相关mRNA相对表达水平的定量分析结果
组别 PCNA CyclinD1 Ki67 对照组 1.000±0.093 1.000±0.153 1.000±0.081 24 h APAP组 0.505±0.0261) 0.390±0.0231) 0.410±0.0441) 48 h APAP组 1.106±0.1432) 0.829±0.0882) 2.009±0.2921)2) F值 20.687 18.744 42.010 P值 0.002 0.003 < 0.001 注:与对照组相比,1)P < 0.01;与24 h APAP组比较,2)P < 0.01。 表 4 AG490对AML12细胞及APAP损伤后的AML12细胞活力的影响
组别 细胞活力 对照组 0.981±0.033 AG490 10 μmol/L组 0.959±0.019 AG490 50 μmol/L组 0.934±0.017 AG490 100 μmol/L组 0.772±0.0101) APAP 2.5 mmol/L组 0.721±0.0161) APAP 2.5 mmol/L+AG490 10 μmol/L组 0.694±0.0141) APAP 2.5 mmol/L+AG490 50 μmol/L组 0.352±0.0211)2) APAP 2.5 mmol/L+AG490 100 μmol/L组 0.164±0.0191)2) F值 685.911 P值 < 0.001 注:与对照组相比,1)P < 0.01;与APAP 2.5 mmol/L组比较,2)P < 0.01。 表 5 Western blot检测AML12细胞中STAT3及增殖相关蛋白表达情况的定量分析结果
组别 p-STAT3/STAT3 PCNA/GAPDH CyclinD1/GAPDH 对照组 1.000±0.032 1.000±0.113 1.000±0.002 APAP组 1.824±0.0561) 0.337±0.068 0.615±0.002 AG490组 0.701±0.0041) 0.971±0.147 0.703±0.001 APAP+AG490组 0.700±0.0331)2) 0.044±0.0032) 0.109±0.0052) F值 211.845 22.963 16 468.823 P值 < 0.001 0.006 < 0.001 注:与对照组相比,1)P < 0.05;与APAP组比较,2)P < 0.05。 表 6 RT-PCR法检测AG490对APAP损伤的AML12细胞中增殖相关基因mRNA的相对表达水平
组别 PCNA CyclinD1 Ki67 对照组 1.000±0.050 1.000±0.138 1.000±0.081 APAP组 1.124±0.084 0.866±0.025 1.968±0.090 APAP+AG490组 0.534±0.0561) 0.354±0.0301) 0.253±0.0351) F值 46.611 33.761 472.386 P值 < 0.001 0.001 < 0.001 注:与APAP组相比,1)P < 0.01。 -
[1] KIM M, YUN JW, SHIN K, et al. Expression levels of GABA-A receptor subunit alpha 3, gabra3 and lipoprotein lipase, lpl are associated with the susceptibility to acetaminophen-induced hepatotoxicity[J]. Biomol Ther (Seoul), 2017, 25(2): 112-121. DOI: 10.4062/biomolther.2016.076 [2] XU LN, LI Y, PENG JY. microRNA and drug-induced liver injury[J]. Chin J Clin Pharmacol Ther, 2020, 25(7): 803-809. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-YLZL202007015.htm许丽娜, 李月, 彭金咏. microRNA与药物性肝损伤[J]. 中国临床药理学与治疗学, 2020, 25(7): 803-809. https://www.cnki.com.cn/Article/CJFDTOTAL-YLZL202007015.htm [3] YAN M, HUO Y, YIN S, et al. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions[J]. Redox Biol, 2018, 17: 274-283. DOI: 10.1016/j.redox.2018.04.019 [4] XIE Y, MCGILL MR, DU K, et al. Mitochondrial protein adducts formation and mitochondrial dysfunction during N-acetyl-m-aminophenol (AMAP)-induced hepatotoxicity in primary human hepatocytes[J]. Toxicol Appl Pharmacol, 2015, 289(2): 213-222. DOI: 10.1016/j.taap.2015.09.022 [5] LIU FC, LEE HC, LIAO CC, et al. Tropisetron protects against acetaminophen-induced liver injury via suppressing hepatic oxidative stress and modulating the activation of JNK/ERK MAPK pathways[J]. Biomed Res Int, 2016, 2016: 1952947. http://pubmedcentralcanada.ca/pmcc/articles/PMC5116490/ [6] ABDULKHALEQ FM, ALHUSSAINY TM, BADR MM, et al. Antioxidative stress effects of vitamins C, E, and B12, and their combination can protect the liver against acetaminophen-induced hepatotoxicity in rats[J]. Drug Des Devel Ther, 2018, 12: 3525-3533. DOI: 10.2147/DDDT.S172487 [7] YU PF, WU Q, DUAN ZP, et al. Research progress in the mechanism of drug-induced liver injury due to paracetamol[J]. J Clin Hepatol, 2019, 35(9): 2108-2112. (in Chinese) DOI: 10.3969/j.issn.1001-5256.2019.09.050余朋飞, 吴桥, 段钟平, 等. 对乙酰氨基酚致药物性肝损伤的机制研究进展[J]. 临床肝胆病杂志, 2019, 35(9): 2108-2112. DOI: 10.3969/j.issn.1001-5256.2019.09.050 [8] BREU AC, PATWARDHAN VR, NAYOR J, et al. A multicenter study into causes of severe acute liver injury[J]. Clin Gastroenterol Hepatol, 2019, 17(6): 1201-1203. DOI: 10.1016/j.cgh.2018.08.016 [9] MOH A, IWAMOTO Y, CHAI GX, et al. Role of STAT3 in liver regeneration: Survival, DNA synthesis, inflammatory reaction and liver mass recovery[J]. Lab Invest, 2007, 87(10): 1018-1028. DOI: 10.1038/labinvest.3700630 [10] ABE M, YOSHIDA T, AKIBA J, et al. STAT3 deficiency prevents hepatocarcinogenesis and promotes biliary proliferation in thioacetamide-induced liver injury[J]. World J Gastroenterol, 2017, 23(37): 6833-6844. DOI: 10.3748/wjg.v23.i37.6833 [11] JUNG J, MOON JW, CHOI JH, et al. Epigenetic alterations of IL-6/STAT3 signaling by placental stem cells promote hepatic regeneration in a rat model with CCl4-induced Liver Injury[J]. Int J Stem Cells, 2015, 8(1): 79-89. DOI: 10.15283/ijsc.2015.8.1.79 [12] RÍO A, GASSULL MA, ALDEGUER X, et al. Reduced liver injury in the interleukin-6 knockout mice by chronic carbon tetrachloride administration[J]. Eur J Clin Invest, 2008, 38(5): 306-316. DOI: 10.1111/j.1365-2362.2008.01939.x [13] HU Z, HAN Y, LIU Y, et al. CREBZF as a key regulator of STAT3 pathway in the control of liver regeneration in mice[J]. Hepatology, 2020, 71(4): 1421-1436. DOI: 10.1002/hep.30919 [14] SIVEEN KS, SIKKA S, SURANA R, et al. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors[J]. Biochim Biophys Acta, 2014, 1845(2): 136-154. http://europepmc.org/abstract/med/24388873 [15] BHUSHAN B, APTE U. Liver regeneration after acetaminophen hepatotoxicity: Mechanisms and therapeutic opportunities[J]. Am J Pathol, 2019, 189(4): 719-729. DOI: 10.1016/j.ajpath.2018.12.006 [16] DENG Y, SHI WH, TONG JD, et al. Effects of epigailocatechin-3-gallate on the proliferation and migration of vascular smooth muscle cells induced by platelet-derived growth factor BB in rats[J]. J Fudan Univ(Med Sci), 2018, 45(4): 503-508. (in Chinese) DOI: 10.3969/j.issn.1672-8467.2018.04.011邓颖, 史伟浩, 童进东, 等. EGCG对血小板源性生长因子-BB诱导的大鼠血管平滑肌细胞增殖和迁移的影响[J]. 复旦学报(医学版), 2018, 45(4): 503-508. DOI: 10.3969/j.issn.1672-8467.2018.04.011 [17] HUANG J, LIU FR, WEN T, et al. Salidroside affects proliferation, invasion and apoptosis of cervical squamous cell carcinoma C33A cells through JAK2/STAT3 pathway[J]. J Chin Cancer Biother, 2020, 27(5): 522 -527. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-ZLSW202005008.htm黄进, 刘福蓉, 温婷, 等. 红景天苷通过JAK2/STAT3通路影响宫颈鳞癌C33A细胞的增殖、侵袭和凋亡[J]. 中国肿瘤生物治疗杂志, 2020, 27(5): 522-527. https://www.cnki.com.cn/Article/CJFDTOTAL-ZLSW202005008.htm [18] KOWALSKA E, BARTNICKI F, FUJISAWA R, et al. Inhibition of DNA replication by an anti-PCNA aptamer/PCNA complex[J]. Nucleic Acids Res, 2018, 46(1): 25-41. DOI: 10.1093/nar/gkx1184 [19] PATEL H, ABDULJABBAR R, LAI CF, et al. Expression of CDK7, Cyclin H, and MAT1 Is elevated in breast cancer and is prognostic in estrogen receptor-positive breast cancer[J]. Clin Cancer Res, 2016, 22(23): 5929-5938. DOI: 10.1158/1078-0432.CCR-15-1104 期刊类型引用(1)
1. 闫红会,张二娟,徐心红. 半坐卧位深部保留灌肠对肝性脑病患者的影响. 内蒙古医学杂志. 2023(11): 1386-1388+1391 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 2009 KB)
PDF下载 ( 2009 KB)


 下载:
下载:
 百度学术
百度学术

