恩替卡韦单药治疗慢性乙型肝炎患者5年HBsAg与HBV DNA的动态变化
DOI: 10.3969/j.issn.1001-5256.2021.05.015
Dynamic changes of HBsAg and hepatitis B virus DNA in chronic hepatitis B patients with entecavir monotherapy for 5 years
-
摘要:
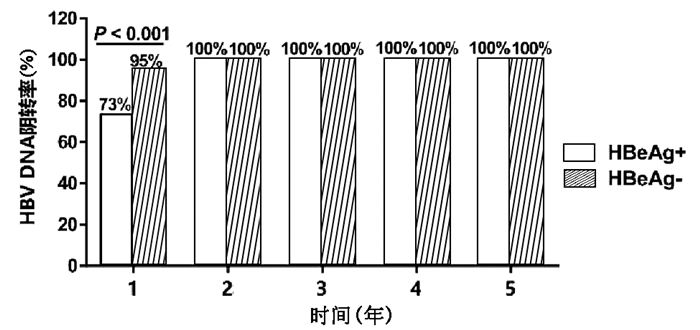
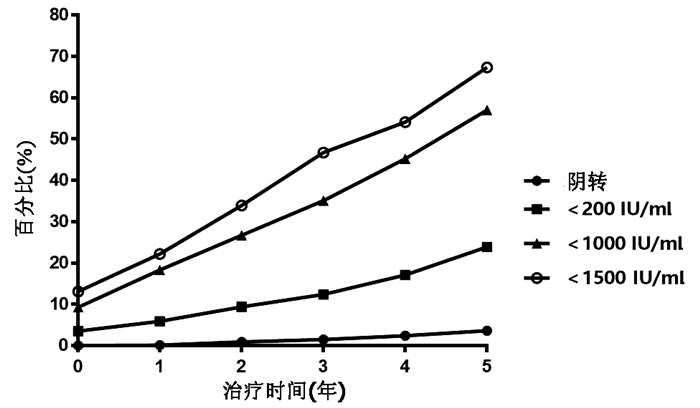
目的 分析慢性乙型肝炎(CHB)患者单用恩替卡韦(ETV)治疗5年血清HBsAg和HBV DNA的动态变化。 方法 回顾性收集2012年1月—2020年8月就诊于西安交通大学第二附属医院的单用ETV治疗≥5年的CHB患者918例,其中初始治疗时HBeAg阳性患者556例,HBeAg阴性患者362例。收集患者在治疗基线、1年、2年、3年、4年、5年时的血清HBsAg水平和HBV DNA载量并分析其动态变化。符合正态分布的计量资料2组间比较采用t检验,不符合正态分布的计量资料2组间比较采用Mann-Whitney U检验;不符合正态分布的重复测量资料先进行log10对数转换,组内或组间比较采用重复测量资料的方差分析;计数资料2组间比较采用χ2检验。 结果 HBeAg阳性和HBeAg阴性患者随着ETV治疗时间的延长,HBsAg水平均呈逐渐下降趋势,组内比较差异有统计学意义(F值分别为174.168、49.845,P值均<0.001)。HBeAg阳性患者基线HBsAg水平为18 043.1(7868.9~26 342.4)IU/ml,ETV治疗5年后降至1397.6 (626.4~3419.4) IU/ml,差异具有统计学意义(P<0.001);HBeAg阳性患者基线HBV DNA载量为(6.7±1.7) log10 IU/ml, ETV治疗5年后HBV DNA阴转率为100%。HBeAg阴性患者的基线HBsAg水平为2420.0(662.6~5621.2)IU/ml,ETV治疗5年后降至407.2 (56.7~1104.7) IU/ml,差异具有统计学意义(P<0.001);HBeAg阴性患者基线HBV DNA载量为(4.9±1.4)log10 IU/ml,ETV治疗5年后HBV DNA阴转率亦为100%。HBeAg阳性患者的中位HBsAg水平在各时间点上均高于HBeAg阴性患者(P值均<0.001)。HBeAg阳性和阴性患者的HBsAg下降均呈现出“先快后慢”的趋势,ETV治疗前两年的HBsAg年均下降速率显著大于后3年(HBeAg阳性: Z=-13.605,P<0.001; HBeAg阴性:Z=-5.950,P<0.001)。HBeAg阳性患者除ETV治疗1年时HBV DNA阴转率显著低于HBeAg阴性患者外(χ2=71.013, P<0.001),其余时间点上两组患者的HBV DNA阴转率均为100%。此外,ETV治疗5年,累计HBsAg转阴、<200 IU/ml、<1000 IU/ml、<1500 IU/ml的患者的比例分别为3.6%、23.9%、57.0%、67.3%。 结论 ETV治疗CHB、HBsAg和HBV DNA水平逐渐下降,并且HBsAg水平的下降呈“先快后慢”的趋势。单用ETV治疗5年,67.3%的患者可以达到HBsAg<1500 IU/ml。 Abstract:Objective To investigate the dynamic changes of serum HBsAg and hepatitis B virus (HBV) DNA in chronic hepatitis B patients (CHB) with entecavir (ETV) monotherapy for 5 years. Methods A retrospective analysis was performed for the clinical data of 918 CHB patients who attended the Second Affiliated Hospital of Xi'an Jiaotong University from January 2012 to August 2020 and received ETV monotherapy for ≥5 years, among whom 556 had positive HBeAg and 362 had negative HBeAg at initial treatment. Serum HBsAg level and HBV DNA load at baseline and at 1, 2, 3, 4, and 5 years of treatment were collected to analyze their dynamic changes. The t-test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups; log10 logarithmic transformation was performed for non-normally distributed repeated measurement data, and then a repeated-measures analysis of variance was used for comparison between groups or within each group; the chi-square test was used for comparison of categorical data between two groups. Results Both HBeAg-positive and HBeAg-negative patients had a significant reduction in HBsAg level over the time of ETV treatment (F=174.168 and 49.845, both P < 0.001). The HBeAg-positive patients had a baseline HBsAg level of 18 043.1 (7868.9-26 342.4) IU/ml, which significantly decreased to 1397.6 (626.4-3419.4) IU/ml after 5 years of ETV treatment (P < 0.001); the HBeAg-positive patients had a baseline HBV DNA load of (6.7±1.7) log10 IU/ml and achieved an HBV DNA clearance rate of 100% after 5 years of ETV treatment. The HBeAg-negative patients had a baseline HBsAg level of 2420.0 (662.6-5621.2) IU/ml, which significantly decreased to 407.2 (56.7-1104.7) IU/ml after 5 years of ETV treatment (P < 0.001); the HBeAg-negative patients had a baseline HBV DNA load of 4.9±1.4 log10 IU/ml and achieved an HBV DNA clearance rate of 100% after 5 years of ETV treatment. The HBeAg-positive patients had a significantly higher median HBsAg level than the HBeAg-negative patients at each time point (all P < 0.001). Both HBeAg-positive and HBeAg-negative patients showed a changing trend of rapid reduction followed by slow reduction in HBsAg, and the mean annual rate of reduction in HBsAg in the first 2 years of ETV treatment was significantly higher than that in the last 3 years of ETV treatment (HBeAg-positive: Z=-13.605, P < 0.001; HBeAg-negative: Z=-5.950, P < 0.001). The HBeAg-positive patients had a significantly lower HBV DNA clearance rate than the HBeAg-negative patients at 1 year of ETV treatment (χ2=71.013, P < 0.001), and both HBeAg-positive and HBeAg-negative patients achieved an HBV DNA clearance rate of 100% at 2, 3, 4, and 5 years of treatment. After 5 years of ETV treatment, the cumulative proportions of patients with HBsAg clearance, HBsAg < 200 IU/ml, HBsAg < 1000 IU/ml, and HBsAg < 1500 IU/ml were 3.6%, 23.9%, 57.0%, and 67.3%, respectively. Conclusion During the EVT treatment of CHB, the levels of HBsAg and HBV DNA gradually decrease, and the level of HBsAg shows a trend of rapid reduction followed by slow reduction. After 5 years of ETV monotherapy, 67.3% of the patients can achieve an HBsAg level of < 1500 IU/ml. -
Key words:
- Hepatitis B, Chronic /
- Hepatitis B Surface Antigens /
- Viral Load /
- Entecavir
-
表 1 患者的基线特征
指标 HBeAg阳性(n=556) HBeAg阴性(n=362) 统计值 P值 性别[例(%)] χ2=3.330 0.073 男 384 (69.1) 229(63.5) 女 172 (30.9) 133(36.7) 年龄(岁) 38.8±11.6 42.8±10.9 t=1.302 0.308 ALT(U/L) 169.9±67.3 153.7±48.7 t=1.312 0.194 AST(U/L) 121.3±13.3 112.4±26.9 t=0.702 0.399 HBsAg(U/ml) 18 043.1(7868.9~26 342.4) 2420.0(662.6~5621.2) Z=-12.665 <0.001 HBV DNA定量(log10 IU/ml) 6.7±1.7 4.9±1.4 t=17.583 <0.001 治疗时间(月) 78.8(69.5~83.6) 75.5(66.3~82.7) Z=3.665 0.032 表 2 HBeAg阳性和阴性CHB患者ETV治疗5年血清HBsAg水平动态变化
组别 例数 基线 1年 2年 3年 4年 5年 F值 P值 HBeAg阳性 556 18 043.1
(7868.9~26 342.4)5893.1
(2742.5~13 265.4)3868.8
(1904.8~8377.8)2620.6
(1387.0~6022.1)1824.9
(923.3~4479.5)1397.6
(626.4~3419.4)174.168 <0.001 HBeAg阴性 362 2420.0
(662.6~5621.2)1244.4
(303.9~2562.5)1038.7
(195.0~2041.9)826.2
(122.9~1674.9)615.7
(88.3~1420.5)407.2
(56.7~1104.7)49.845 <0.001 注:各时间点组间比较,P<0.001;组内各时间点两两比较,P<0.001。 表 3 不同水平基线HBsAg的HBeAg阳性患者HBsAg水平的5年动态变化
HBsAg水平 例数 基线 1年 2年 3年 4年 5年 F值 P值 <3000 IU/ml 62 1262.1
(692.0~2251.9)899.7
(377.7~2190.0)708.5
(295.4~2046.3)619.1
(190.5~1639.4)435.9
(104.3~1290.4)320.8
(80.3~826.4)22.201 <0.001 3000~10 000 IU/ml 122 6982.6
(5282.5~8385.2)2968.2
(1890.3~3758.7)2116.8
(1576.3~2814.3)1787.7
(908.7~2442.9)1410.2
(759.9~1835.6)1025.9
(560.1~1613.8)37.877 <0.001 10 000~20 000 IU/ml 108 15 317.0
(12 412.7~16 385.5)6 027.47
(3596.4~7740.7)3947.0
(2091.0~5577.7)2610.0
(1083.7~4371.8)1633.3
(787.5~3917.5)1491.7
(618.2~3081.9)51.770 <0.001 ≥20 000 IU/ml 264 26 691.1
(25 000.0~38 816.0)13 290.5
(7383.8~18 600.0)8304.3
(4258.5~14 090.0)5672.0
(2649.1~9325.9)3929.3
(1623.4~7272.5)2682.0
(1065.0~5402.4)136.124 <0.001 表 4 不同水平基线HBsAg的HBeAg阴性患者HBsAg水平的5年动态变化
HBsAg水平 例数 基线 1年 2年 3年 4年 5年 F值 P值 <3000 IU/ml 166 749.3
(222.4~1913.5)363.8
(80.5~1014.1)300.5
(49.0~955.9)233.9
(37.0~808.7)149.8
(29.3~632.1)79.6
(14.3~420.5)22.866 <0.001 3000~10 000 IU/ml 128 5552.4
(3709.9~7075.3)2400.0
(1690.5~3835.6)1928.9
(1273.2~2846.1)1483.0
(868.5~2422.4)1126.3
(629.9~1924.7)928.1
(411.9~1666.9)33.684 <0.001 10 000~20 000 IU/ml 35 13 142.5
(11 578.7~15 600.7)6937.0
(5495.0~11 289.5)4980.7
(4219.5~9006.2)4410.1
(2447.4~6832.0)3775.1
(1985.5~5372.1)2731.9
(1682.2~4097.9)21.129 <0.001 ≥20 000 IU/ml 33 22 190.4
(21 004.2~29 850.9)11 390.3
(5283.5~14 280.2)6536.4
(2933.2~9240.4)4694.0
(2622.7~8037.3)4236.3
(2304.2~6944.9)2902.3
(1429.5~6030.2)16.562 <0.001 表 5 不同时间点HBeAg阳性和HBeAg阴性CHB患者的HBsAg分布
HBsAg水平 1年 2年 3年 4年 5年 <0.05 IU/ml HBeAg阳性 1(0.2) 6(1.1) 9(1.6) 14(2.5) 19(3.4) HBeAg阴性 0 2(0.6) 5(1.4) 8(2.2) 14(3.9) χ2值 0 0.226 0.082 0.089 0.128 P值 1.000 0.490 0.794 0.829 0.856 <200 IU/ml HBeAg阳性 24(4.3) 43(7.8) 51(9.2) 71(12.8) 85(15.3) HBeAg阴性 30(8.3) 43(11.9) 63 (17.4) 86(23.8) 134(37.1) χ2值 6.244 4.436 13.656 18.669 56.987 P值 0.015 0.037 <0.001 <0.001 <0.001 <1000 IU/ml HBeAg阳性 76(13.7) 102(18.3) 138(24.8) 177(31.8) 258(46.4) HBeAg阴性 92(25.4) 143(39.5) 184(50.8) 238(65.7) 265(73.2) χ2值 20.229 50.162 65.126 101.789 64.246 P值 <0.001 <0.001 <0.001 <0.001 <0.001 <1500 IU/ml HBeAg阳性 98(17.6) 135(24.3) 196(35.3) 243(43.7) 341(61.3) HBeAg阴性 106(29.3) 176(48.6) 233(64.4) 254(70.2) 277(76.5) χ2值 17.234 57.977 74.650 61.829 22.990 P值 <0.001 <0.001 <0.001 <0.001 <0.001 -
[1] Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [2] ZHAO Y, LI Y, QI L, et al. Antiviral curative effects of tenofovir and entecavir in treatment of aged patients with chronic hepatitis B and their regulation on inflammation factors[J]. J Jilin Univ(Med Edit), 2019, 45(1): 117-122. DOI: 10.13481/j.1671-587x.20190122.赵阳, 李烨, 齐玲, 等. 替诺福韦酯和恩替卡韦治疗老年慢性乙型肝炎患者的抗病毒疗效及对致炎细胞因子的调节作用[J]. 吉林大学学报(医学版), 2019, 45(1): 117-122. DOI: 10.13481/j.1671-587x.20190122. [3] LIN L, LIU JY, DENG H, et al. Influencing factors of partial virological response to entecavir monotherapy of patients with chronic hepatitis B[J/CD]. Chin J Liver Dis(Electronic Edition), 2019, 11(4): 81-84. DOI: 10.3969/j.issn.1674-7380.2019.04.013.林丽, 刘俊英, 邓慧, 等. 恩替卡韦单药治疗慢性乙型肝炎患者部分病毒学应答的影响因素[J/CD]. 中国肝脏病杂志(电子版), 2019, 11(4): 81-84. DOI: 10.3969/j.issn.1674-7380.2019.04.013. [4] XU Y, ZHANG YG, WANG X, et al. Long-term antiviral efficacy of entecavir and liver histology improvement in Chinese patients with hepatitis B virus-related cirrhosis[J]. World J Gastroenterol, 2015, 21(25): 7869-7876. DOI: 10.3748/wjg.v21.i25.7869. [5] SETO WK, WONG DK, FUNG J, et al. Reduction of hepatitis B surface antigen levels and hepatitis B surface antigen seroclearance in chronic hepatitis B patients receiving 10 years of nucleoside analogue therapy[J]. Hepatology, 2013, 58(3): 923-931. DOI: 10.1002/hep.26376. [6] FAN XX, CUI FM, WANG FS, et al. Analysis of the characteristics and influencing factors of HBsAg change in chronic hepatitis B patients treated with NAs[J]. Acta Acad Med Bengbu, 2019, 44(5): 582-584, 589. DOI: 10.13898/j.cnki.issn.1000-2200.2019.05.005.范新星, 崔甫朦, 王福生, 等. NAs治疗慢性乙型肝炎后HBsAg变化的特点和影响因素分析[J]. 蚌埠医学院学报, 2019, 44(5): 582-584, 589. DOI: 10.13898/j.cnki.issn.1000-2200.2019.05.005. [7] XI HL, LI MR, BAO Y, et al. Antiviral dynamic change of HBV DNA and HBsAg and significance of quarterly and annual quantitative measurements over 5 years follow up of chronic hepatitis B patient[J]. Chin J Hepatol, 2013, 21(11): 821-824. DOI: 10.3760/cma.j.issn.1007-3418.2013.11.006.席宏丽, 李敏然, 鲍毅, 等. 核苷(酸)类抗病毒药物治疗慢性乙型肝炎患者HBV DNA与HBsAg定量的5年动态分析[J]. 中华肝脏病杂志, 2013, 21(11): 821-824. DOI: 10.3760/cma.j.issn.1007-3418.2013.11.006. [8] CHU CJ, HUSSAIN M, LOK AS. Quantitative serum HBV DNA levels during different stages of chronic hepatitis B infection[J]. Hepatology, 2002, 36(6): 1408-1415. DOI: 10.1053/jhep.2002.36949. [9] HSU WF, CHEN CF, LAI HC, et al. Trajectories of serum hepatitis B surface antigen kinetics in patients with chronic hepatitis B receiving long-term nucleos(t)ide analogue therapy[J]. Liver Int, 2018, 38(4): 627-635. DOI: 10.1111/liv.13564. [10] European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021. [11] TAN L, WU T, LV Y, et al. HBsAg levels normalized to the same hepatic parenchyma cell volume is correlated with pathological progression but not HBeAg status[J]. J Sun Yat-Sen Univ(Medical Sciences), 2019, 40(5): 747-753. DOI: 10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).2019.0104.谭雷, 吴涛, 吕艳, 等. 经相同肝实质细胞体积分摊的血清HBsAg水平与肝组织病理进展相关而与HBeAg状态无关[J]. 中山大学学报(医学科学版), 2019, 40(5): 747-753. DOI: 10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).2019.0104. [12] CHEN CH, LU SN, HUNG CH, et al. The role of hepatitis B surface antigen quantification in predicting HBsAg loss and HBV relapse after discontinuation of lamivudine treatment[J]. J Hepatol, 2014, 61(3): 515-522. DOI: 10.1016/j.jhep.2014.04.029. [13] SETO WK, LIU K, WONG DK, et al. Patterns of hepatitis B surface antigen decline and HBV DNA suppression in Asian treatment-experienced chronic hepatitis B patients after three years of tenofovir treatment[J]. J Hepatol, 2013, 59(4): 709-716. DOI: 10.1016/j.jhep.2013.06.007. [14] CHEN JL, YUAN ZH. Influence of interferon and nucleos(t)ide analogues on HBV cccDNA and functional cure of chronic hepatitis B[J]. J Clin Hepatol, 2019, 35(6): 1181-1187. DOI: 10.3969/j.issn.1001-5256.2019.06.002.陈捷亮, 袁正宏. 干扰素和核苷(酸)类似物治疗对HBV cccDNA的影响与慢性乙型肝炎的功能性治愈[J]. 临床肝胆病杂志, 2019, 35(6): 1181-1187. DOI: 10.3969/j.issn.1001-5256.2019.06.002. [15] Chinese Medical Association, Chinese Society of Infectious Disease, Chinese Society of Hepatology, Chinese Medical Association. The expert consensus on clinical cure (functional cure) of chronic hepatitis B[J]. J Clin Hepatol, 2019, 35(8): 1693-1701. DOI: 10.3969/j.issn.1001-5256.2019.08.008.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎临床治愈(功能性治愈)专家共识[J]. 临床肝胆病杂志, 2019, 35(8): 1693-1701. DOI: 10.3969/j.issn.1001-5256.2019.08.008. [16] ZHOU YQ, LI C, YIN FM, et al. Change in HBsAg quantification after HBV DNA negative conversion in chronic hepatitis B patients treated with nucleos(t)ide analogues[J]. J Clin Hepatol, 2019, 35(5): 977-981. DOI: 10.3969/j.issn.1001-5256.2019.05.009.周友乾, 李翠, 尹凤鸣, 等. 核苷(酸)类似物治疗慢性乙型肝炎患者HBV DNA阴转后HBsAg定量的变化[J]. 临床肝胆病杂志, 2019, 35(5): 977-981. DOI: 10.3969/j.issn.1001-5256.2019.05.009. -



 PDF下载 ( 1991 KB)
PDF下载 ( 1991 KB)

 下载:
下载: