阿司匹林对病毒性肝炎患者肝癌发生率影响的Meta分析
DOI: 10.3969/j.issn.1001-5256.2021.05.024
Effect of aspirin on the incidence rate of liver cancer in patients with viral hepatitis: A Meta-analysis
-
摘要:
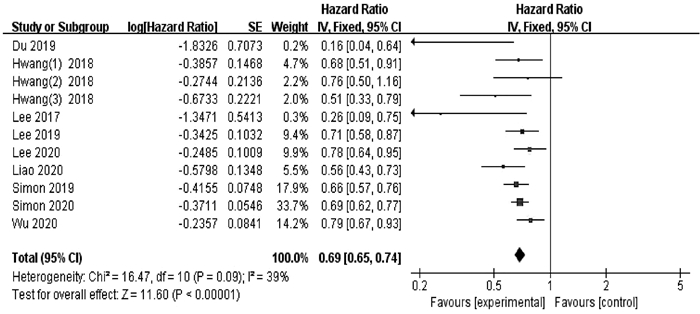
目的 评价阿司匹林的使用对病毒性肝炎患者肝癌发生率的影响。 方法 检索PubMed、Web of Science、Cochrane Library英文数据库,收集建库至2020年7月15日关于病毒性肝炎患者使用阿司匹林与肝癌发生率的研究。选择风险比(HR)和95%CI作为汇总指标。用RevMan 5.3进行Meta分析、对不能合并的数据采用描述性分析。 结果 共纳入9篇文献,涉及132 066例病毒性肝炎患者。结果表明使用阿司匹林的病毒性肝炎患者肝癌发生率降低31%(HR=0.69,95%CI: 0.65~0.74,P<0.000 01);4项研究报道了消化道出血发生率,表明使用阿司匹林未显著增加病毒性肝炎患者消化道出血风险(P>0.05)。 结论 接受阿司匹林治疗可能有利于降低病毒性肝炎患者肝癌发生率,未显著增加包括肝硬化人群在内的消化道出血风险,对于减少肝癌的发生可能具有积极意义,但该临床现象背后的机制尚不明确,且仍需更多临床观察以验证其安全性和有效性。 -
关键词:
- 肝炎, 病毒性, 人 /
- 阿司匹林 /
- 癌, 肝细胞 /
- Meta分析(主题)
Abstract:Objective To evaluate the effect of the use of aspirin on the incidence rate of liver cancer in patients with viral hepatitis. Methods English databases including PubMed, Web of Science, and Cochrane Library were searched for studies on the use of aspirin and the incidence rate of liver cancer in patients with viral hepatitis published up to July 15, 2020. Hazard ratio (HR) and 95% confidence interval (CI) were selected as pooled indicators. RevMan 5.3 was used to perform the Meta-analysis, and a descriptive analysis was performed for data that could not be pooled. Results Nine studies were included, involving 132 066 patients with viral hepatitis. The results showed that the incidence rate of liver cancer was reduced by 31% in the patients with viral hepatitis who were treated with aspirin (HR=0.69, 95% CI: 0.65-0.74, P < 0.000 01). Four studies reported the incidence rate of gastrointestinal bleeding, suggesting that the use of aspirin did not significantly increase the risk of gastrointestinal bleeding in patients with viral hepatitis(P > 0.05). Conclusion Aspirin therapy may help to reduce the incidence rate of liver cancer in patients with viral hepatitis and does not significantly increase the risk of gastrointestinal bleeding in such patients and the patients with liver cirrhosis. Aspirin may have a positive significance in reducing liver cancer, but the mechanism behind this clinical phenomenon remains unclear, and therefore, more clinical observation studies are needed to verify its safety and efficacy. -
Key words:
- Hepatitis, Viral, Human /
- Aspirin /
- Carcinoma, Hepatocellular /
- Meta-Analysis as Topic
-
表 1 Meta分析纳入文献的基本特征
纳入研究 地区 研究类型 随访时间 阿司匹林使用定义 随访人数 人群 Simon等[21](2020) 瑞典 队列研究 中位随访期7.9年 低剂量(75 mg或160 mg),≥90 DDDs 50 275 HBV/HCV Liao等[22](2020) 中国台湾 队列研究 最长随访期≥12年 NA 3822 HCV Wu等[23](2020) 中国台湾 队列研究 最长随访期≥5年 ≥90 DDDs 9417 HCV Lee等[24](2020) 中国台湾 队列研究 最长随访期≥5年 规律用药≥90 d 7434 HCV Lee等[25](2019) 中国台湾 队列研究 最长随访期≥5年 规律用药≥90 d 10 615 HBV Simon等[26](2019) 瑞典 队列研究 中位随访期8.5年 剂量<162 mg且≥30 DDDs 18 368 HBV/HCV Du等[27](2019) 中国 队列研究 中位随访期4.5年 术后7 d内开始服用,剂量为100 mg/d且持续≥1年 264 HBV/HCV Hwang等[28](2018) 韩国 队列研究 中位随访期4年 ≥30 DDDs 31 528 病毒性肝炎 Lee等[29](2017) 韩国 队列研究 中位随访期38.5个月 100 mg/d,使用时间≥6个月 343 HBV 纳入研究 是否包含肝硬化人群 是否抗病毒治疗 匹配或调整变量 报告结局 NOS评分 Simon等[21](2020) 是。对照组14.2%,阿司匹林组13.6% 是。对照组31.7%,阿司匹林组29.2% 倾向性评分;2~8 ①② 9 Liao等[22](2020) 是。NA 否 倾向性评分;1、2、5~7 ① 9 Wu等[23](2020) 是。NA 是。对照组5.4%,阿司匹林组5.5% 倾向性评分;1、2、4~8 ① NA Lee等[24](2020) 是。对照组15.1%,阿司匹林组16.0% 是。对照组4.7%,阿司匹林组5.4% 倾向性评分;1、2、4~8 ①② 9 Lee等[25](2019) 是。对照组17.1%,阿司匹林组17.1% 是。对照组15.8%,阿司匹林组15.8% 倾向性评分;1~3、5~8 ①② 9 Simon等[26](2019) NA NA 倾向性评分;NA ① NA Du等[27](2019) 是。对照组100%,阿司匹林组100% NA NA ① 7 Hwang等[28](2018) NA NA 1、2、7、8 ① 8 Lee等[29](2017) 是。阿司匹林组12.2% 否 倾向性评分;1、5、8 ①② 8 注:调整变量1~8分别为1,年龄;2,性别;3,抗HBV治疗;4,抗HCV治疗;5,肝硬化;6,其他药物(如二甲双胍、他汀类等具有潜在化学预防肝癌药物);7,合并症;8,其他。报告结局包括①肝癌发生率;②消化道出血发生率。DDDs,限定日剂量。NA, 未提及。 表 2 亚组分析
亚组 研究数目 HR(95%CI) P值 I2值(%) P值 地域 东方[22-25, 27-29] 7 0.67(0.58~0.78) <0.000 1 50 0.04 西方[21, 26] 2 0.68(0.62~0.74) <0.000 1 0 0.63 肝炎类型 HBV[25, 29] 2 0.49(0.19~1.27) 0.14 70 0.07 HCV[22-24] 3 0.72(0.59~0.87) <0.000 1 60 0.08 混合病例[21, 26-28] 4 0.67(0.62~0.73) <0.000 1 20 0.28 人群 抗病毒治疗[23-25] 3 0.68(0.42~1.09) 0.11 46 0.16 肝硬化[23-25, 27] 4 0.77(0.58~1.02) 0.07 53 0.09 匹配或调整变量 抗病毒治疗[21, 23-25] 4 0.73(0.67~0.78) <0.000 1 0 0.49 肝硬化[21-25, 29] 6 0.71(0.66~0.76) <0.000 1 46 0.10 -
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. [2] BAECKER A, LIU X, LA VECCHIA C, et al. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors[J]. Eur J Cancer Prev, 2018, 27(3): 205-212. DOI: 10.1097/CEJ.0000000000000428. [3] LOK AS, MCMAHON BJ, BROWN RS Jr, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis[J]. Hepatology, 2016, 63(1): 284-306. DOI: 10.1002/hep.28280. [4] BANG CS, SONG IH. Impact of antiviral therapy on hepatocellular carcinoma and mortality in patients with chronic hepatitis C: Systematic review and meta-analysis[J]. BMC Gastroenterol, 2017, 17(1): 46. DOI: 10.1186/s12876-017-0606-9. [5] TONG MJ, THEODORO CF, SALVO RT. Late development of hepatocellular carcinoma after viral clearance in patients with chronic hepatitis C: A need for continual surveillance[J]. J Dig Dis, 2018, 19(7): 411-420. DOI: 10.1111/1751-2980.12615. [6] NA SK, SONG BC. Development and surveillance of hepatocellular carcinoma in patients with sustained virologic response after antiviral therapy for chronic hepatitis C[J]. Clin Mol Hepatol, 2019, 25(3): 234-244. DOI: 10.3350/cmh.2018.0108. [7] PIEPOLI MF, HOES AW, AGEWALL S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The sixth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts)developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR)[J]. Eur Heart J, 2016, 37(29): 2315-2381. DOI: 10.1093/eurheartj/ehw106. [8] KINOSHITA M, YOKOTE K, ARAI H, et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017[J]. J Atheroscler Thromb, 2018, 25(9): 846-984. DOI: 10.5551/jat.GL2017. [9] BIBBINS-DOMINGO K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. preventive services task force recommendation statement[J]. Ann Intern Med, 2016, 164(12): 836-845. DOI: 10.7326/M16-0577. [10] BOSETTI C, SANTUCCI C, GALLUS S, et al. Aspirin and the risk of colorectal and other digestive tract cancers: An updated meta-analysis through 2019[J]. Ann Oncol, 2020, 31(5): 558-568. DOI: 10.1016/j.annonc.2020.02.012. [11] HUA H, ZHANG H, KONG Q, et al. Complex roles of the old drug aspirin in cancer chemoprevention and therapy[J]. Med Res Rev, 2019, 39(1): 114-145. DOI: 10.1002/med.21514. [12] HOSSAIN MA, KIM DH, JANG JY, et al. Aspirin enhances doxorubicin-induced apoptosis and reduces tumor growth in human hepatocellular carcinoma cells in vitro and in vivo[J]. Int J Oncol, 2012, 40(5): 1636-1642. DOI: 10.3892/ijo.2012.1359. [13] PETRICK JL, SAHASRABUDDHE VV, CHAN AT, et al. NSAID use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project[J]. Cancer Prev Res (Phila), 2015, 8(12): 1156-1162. DOI: 10.1158/1940-6207.CAPR-15-0126. [14] SAHASRABUDDHE VV, GUNJA MZ, GRAUBARD BI, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma[J]. J Natl Cancer Inst, 2012, 104(23): 1808-1814. DOI: 10.1093/jnci/djs452. [15] SIMON TG, MA Y, LUDVIGSSON JF, et al. Association between aspirin use and risk of hepatocellular carcinoma[J]. JAMA Oncol, 2018, 4(12): 1683-1690. DOI: 10.1001/jamaoncol.2018.4154. [16] YANG B, PETRICK JL, CHEN J, et al. Associations of NSAID and paracetamol use with risk of primary liver cancer in the Clinical Practice Research Datalink[J]. Cancer Epidemiol, 2016, 43: 105-111. DOI: 10.1016/j.canep.2016.06.009. [17] SHIN S, LEE SH, LEE M, et al. Aspirin and the risk of hepatocellular carcinoma development in patients with alcoholic cirrhosis[J]. Medicine (Baltimore), 2020, 99(9): e19008. DOI: 10.1097/MD.0000000000019008. [18] WANG S, YU Y, RYAN PM, et al. Association of aspirin therapy with risk of hepatocellular carcinoma: A systematic review and dose-response analysis of cohort studies with 2.5 million participants[J]. Pharmacol Res, 2020, 151: 104585. DOI: 10.1016/j.phrs.2019.104585. [19] TAO Y, LI Y, LIU X, et al. Nonsteroidal anti-inflammatory drugs, especially aspirin, are linked to lower risk and better survival of hepatocellular carcinoma: A meta-analysis[J]. Cancer Manag Res, 2018, 10: 2695-2709. DOI: 10.2147/CMAR.S167560. [20] PANG Q, JIN H, QU K, et al. The effects of nonsteroidal anti-inflammatory drugs in the incident and recurrent risk of hepatocellular carcinoma: A meta-analysis[J]. Onco Targets Ther, 2017, 10: 4645-4656. DOI: 10.2147/OTT.S143154. [21] SIMON TG, DUBERG AS, ALEMAN S, et al. Association of aspirin with hepatocellular carcinoma and liver-related mortality[J]. N Engl J Med, 2020, 382(11): 1018-1028. DOI: 10.1056/NEJMoa1912035. [22] LIAO YH, HSU RJ, WANG TH, et al. Aspirin decreases hepatocellular carcinoma risk in hepatitis C virus carriers: A nationwide cohort study[J]. BMC Gastroenterol, 2020, 20(1): 6. DOI: 10.1186/s12876-020-1158-y. [23] WU CY, LEE TY, HSU YC, Ho HJ, et al. Association of daily aspirin therapy with hepatocellular carcinoma risk in patients with chronic hepatitis C[J]. Gastroenterology, 2020, 158(6): s1294. http://www.sciencedirect.com/science/article/pii/S1542356520305334 [24] LEE TY, HSU YC, TSENG HC, et al. Association of daily aspirin therapy with hepatocellular carcinoma risk in patients with chronic hepatitis C virus infection[J]. Clin Gastroenterol Hepatol, 2020, 18(12): 2784-2792. e7. DOI: 10.1016/j.cgh.2020.04.036. [25] LEE TY, HSU YC, TSENG HC, et al. Association of daily aspirin therapy with risk of hepatocellular carcinoma in patients with chronic hepatitis B[J]. JAMA Intern Med, 2019, 179(5): 633-640. DOI: 10.1001/jamainternmed.2018.8342. [26] SIMON TG, DUBERG AS, ALEMAN S, et al. Aspirin use is associated with reduced risk for incident hepatocellular carcinoma in patients with chronic viral hepatitis: Result from a nantionwide population[J]. Gastroenterology, 2019, 156(6): s1200. http://www.researchgate.net/publication/332924969_496_-_Aspirin_Use_is_Associated_with_Reduced_Risk_for_Incident_Hepatocellular_Carcinoma_in_Patients_with_Chronic_Viral_Hepatitis_Results_from_a_Nationwide_Population [27] DU ZQ, ZHAO JZ, DONG J, et al. Effect of low-dose aspirin administration on long-term survival of cirrhotic patients after splenectomy: A retrospective single-center study[J]. World J Gastroenterol, 2019, 25(28): 3798-3807. DOI: 10.3748/wjg.v25.i28.3798. [28] HWANG IC, CHANG J, KIM K, et al. Aspirin use and risk of hepatocellular carcinoma in a national cohort study of Korean Adults[J]. Sci Rep, 2018, 8(1): 4968. DOI: 10.1038/s41598-018-23343-0. [29] LEE M, CHUNG GE, LEE JH, et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment[J]. Hepatology, 2017, 66(5): 1556-1569. DOI: 10.1002/hep.29318. [30] El-SERAG HB. Epidemiology of viral hepatitis and hepatocellular carcinoma[J]. Gastroenterology, 2012, 142(6): 1264-1273. e1261. DOI: 10.1053/j.gastro.2011.12.061. [31] ARAVALLI RN, STEER CJ, CRESSMAN ENK. Molecular mechanisms of hepatocellular carcinoma[J]. Hepatology, 2008, 48(6): 2047-2063. DOI: 10.1002/hep.22580. [32] COFFELT SB, de VISSER KE. Cancer: Inflammation lights the way to metastasis[J]. Nature, 2014, 507(7490): 48-49. DOI: 10.1038/nature13062. [33] ELWOOD PC, MORGAN G, PICKERING JE, et al. Aspirin in the treatment of cancer: Reductions in metastatic spread and in mortality: A systematic review and meta-analyses of published studies[J]. PLoS One, 2016, 11(4): e0152402. DOI: 10.1371/journal.pone.0152402. [34] SMITH WL, GARAVITO RM, DEWITT DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2[J]. J Biol Chem, 1996, 271(52): 33157-33160. DOI: 10.1074/jbc.271.52.33157. [35] CHEN H, CAI W, CHU E, et al. Hepatic cyclooxygenase-2 overexpression induced spontaneous hepatocellular carcinoma formation in mice[J]. Oncogene, 2017, 36(31): 4415-4426. DOI: 10.1038/onc.2017.73. [36] CHEN G, LI X, YANG J, et al. Prognostic significance of cyclooxygenase-2 expression in patients with hepatocellular carcinoma: A meta-analysis[J]. Arch Med Sci, 2016, 12(5): 1110-1117. DOI: 10.5114/aoms.2016.61916. [37] HWANG SJ, LUO JC, LI CP, et al. Thrombocytosis: A paraneoplastic syndrome in patients with hepatocellular carcinoma[J]. World J Gastroenterol, 2004, 10(17): 2472-2477. DOI: 10.3748/wjg.v10.i17.2472. [38] SIMANEK R, VORMITTAG R, AY C, et al. High platelet count associated with venous thromboembolism in cancer patients: Results from the Vienna Cancer and Thrombosis Study (CATS)[J]. J Thromb Haemost, 2010, 8(1): 114-120. DOI: 10.1111/j.1538-7836.2009.03680.x. [39] GAY LJ, FELDING-HABERMANN B. Contribution of platelets to tumour metastasis[J]. Nat Rev Cancer, 2011, 11(2): 123-134. DOI: 10.1038/nrc3004. [40] SITIA G, AIOLFI R, DI LUCIA P, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B[J]. Proc Natl Acad Sci U S A, 2012, 109(32): e2165-e2172. DOI: 10.1073/pnas.1209182109. [41] HSU CS, CHAO YC, LIN HH, et al. Systematic review: Impact of interferon-based therapy on HCV-related hepatocellular carcinoma[J]. Sci Rep, 2015, 5: 9954. DOI: 10.1038/srep09954. [42] GARCÍA RODRÍGUEZ LA, MARTÍN-PÉREZ M, HENNEKENS CH, et al. Bleeding risk with long-term low-dose aspirin: A systematic review of observational studies[J]. PLoS One, 2016, 11(8): e0160046. DOI: 10.1371/journal.pone.0160046. [43] TANTAI XX, YANG LB, WEI ZC, et al. Association of proton pump inhibitors with risk of hepatic encephalopathy in advanced liver disease: A meta-analysis[J]. World J Gastroenterol, 2019, 25(21): 2683-2698. DOI: 10.3748/wjg.v25.i21.2683. [44] SCHWEITZER A, HORN J, MIKOLAJCZYK RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013[J]. Lancet, 2015, 386(10003): 1546-1555. DOI: 10.1016/S0140-6736(15)61412-X. -



 PDF下载 ( 2235 KB)
PDF下载 ( 2235 KB)

 下载:
下载: