AU结合因子1对肝细胞癌患者磷脂酰肌醇蛋白聚糖3水平的影响
DOI: 10.3969/j.issn.1001-5256.2021.05.027
利益冲突声明:本研究不存在研究者、伦理委员会成员、受试者监护人以及与公开研究成果有关的利益冲突。
作者贡献声明:张婷负责课题的实施与论文撰写;关贵文、张婧负责数据库分析与图表整理;鲁凤民参与修改完善论文;陈香梅负责课题设计,指导撰写文章并最后定稿。
Effect of AU-rich element RNA-binding factor 1 on the level of glypican 3 in hepatocellular carcinoma
-
摘要:
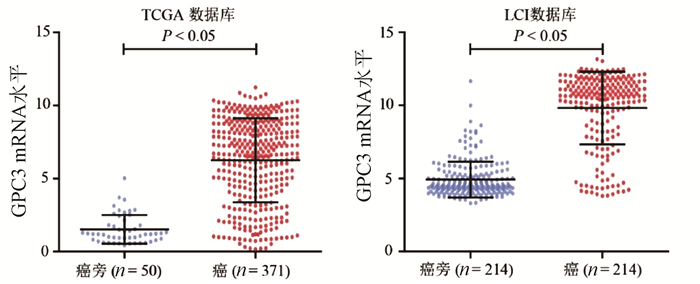
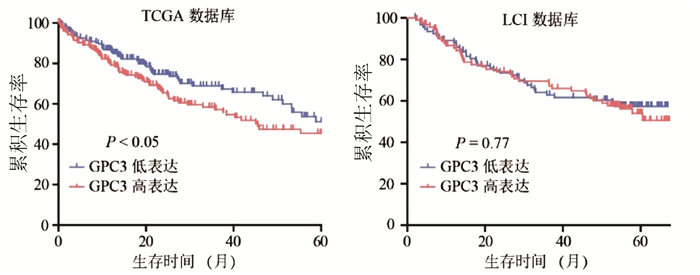
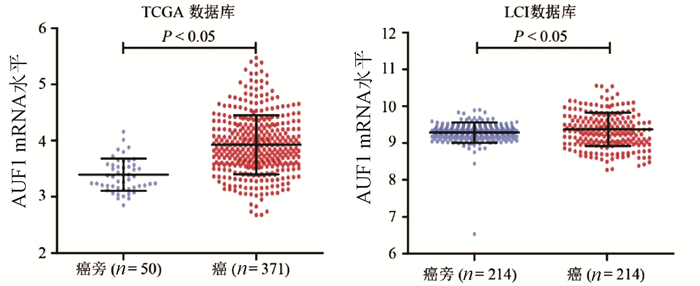
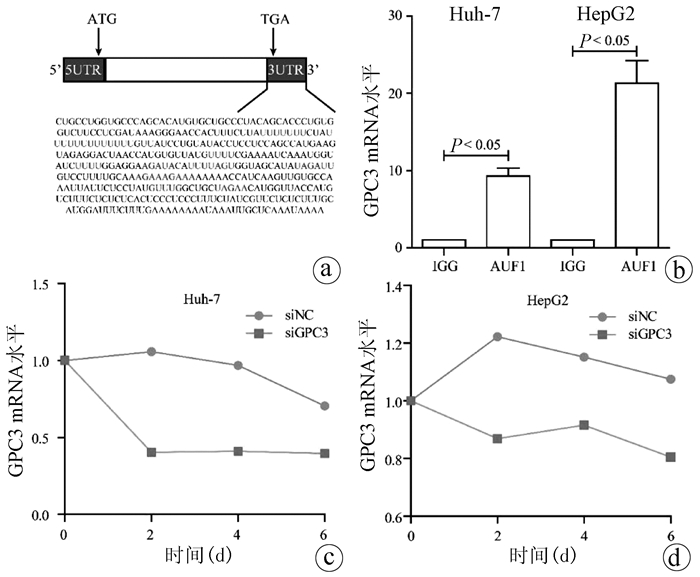
目的 探讨肝细胞癌(HCC)患者中AU结合因子1(AUF1)对磷脂酰肌醇蛋白聚糖3(GPC3)表达的影响及其机制。 方法 TCGA-HCC基因表达数据下载自美国Bead研究所基因数据分析中心, 最终纳入371例不同病因的HCC组织及50例癌旁组织; LCI-HCC基因表达数据下载自GSE14520, 最终纳入214例有随访信息的乙型肝炎相关HCC患者。35例HCC及配对癌旁样本选取自2009年—2013年在河南省肿瘤医院接受常规根治性手术的HCC患者。采用免疫组化检测GPC3和AUF1蛋白在HCC中的表达及相关性;在HCC细胞系中敲减或者过表达AUF1后,采用Western Blot和实时荧光定量PCR检测GPC3表达;利用RNA结合蛋白免疫沉淀实验和RNA稳定性检测研究AUF1调控GPC3表达的机制。计量资料两组间比较采用t检验,计数资料两组间比较采用χ2检验,Kaplan-Meier方法应用于术后患者的生存分析,并使用log-rank检验进行生存率的比较。 结果 在TCGA和LCI数据库中,肝癌组织GPC3的表达均显著高于癌旁组织(P值均<0.05);TCGA数据库中,GPC3高表达与肝癌患者的不良预后有关(P<0.05)。免疫组化结果显示,GPC3和AUF1蛋白均在肝癌组织中呈高表达,阳性表达率分别为77.1%(27/35)和74.3%(26/35)。体外实验结果表明,在肝癌细胞系HepG2和Huh-7中敲减AUF1明显降低了GPC3表达(P值均<0.05),而过表达AUF1则上调GPC3表达(P<0.05)。AUF1蛋白可与GPC3 mRNA结合,敲减AUF1导致GPC3 mRNA稳定性降低。 结论 AUF1是调控GPC3基因转录后修饰的重要调节因子,AUF1和GPC3蛋白的异常高表达可能参与肝癌的发生和发展。 -
关键词:
- 癌, 肝细胞 /
- AU结合因子1 /
- 磷脂酰肌醇蛋白聚糖3
Abstract:Objective To investigate the effect of AU-rich element RNA-binding factor 1 (AUF1) on glypican 3 (GPC3) in hepatocellular carcinoma (HCC) and its possible mechanism. Methods TCGA-HCC gene expression data were downloaded from Broad Institute Genome Data Analysis Center, and finally 371 HCC tissue samples with different etiologies and 50 adjacent tissue samples were included; LCI-HCC gene expression data were downloaded from GSE14520, and 214 patients with hepatitis B-associated HCC who had follow-up data were enrolled. A total of 35 primary liver cancer samples and corresponding adjacent tissue samples were collected from HCC patients who underwent radical surgery in Henan Provincial Cancer Hospital from 2009 to 2013. Immunohistochemistry was used to measure the protein expression of GPC3 and AUF1 in HCC tissue; Western Blot and qRT-PCR were used to measure the expression of GPC3 after AUF1 knockdown or overexpression in hepatoma cell lines; RNA-binding protein immunoprecipitation and RNA turnover assay were used to investigate the potential mechanism of AUF1 in regulating the expression of GPC3. The t-test was used for comparison of quantitative data between two groups, and the chi-square test was used for comparison of rates between two groups; the Kaplan-Meier method was used for survival analysis after surgery, and the log-rank test was used for comparison of survival rates. Results In TCGA and LCI databases, the expression of GPC3 in HCC tissue was significantly higher than that in adjacent tissue (P < 0.05), and in TCGA database, the high expression of GPC3 was associated with the poor prognosis of HCC patients (P < 0.05). Immunohistochemistry showed that both GPC3 and AUF1 proteins are highly expressed in HCC tissue, with a positive expression rate of 77.1% (27/35) and 74.3% (26/35), respectively. In vitro experiment showed that AUF1 knockdown significantly reduced the expression of GPC3 in HepG2 and Huh-7 cells (P < 0.05), while AUF1 overexpression significantly increased the expression of GPC3 (P < 0.05). AUF1 protein could bind to GPC3 mRNA, and AUF1 knockdown reduced the stability of GPC3 mRNA. Conclusion AUF1 is an important post-transcriptional regulator of the GPC3 gene, and the abnormal high expression of AUF1 and GPC3 may be involved in the development and progression of HCC. -
[1] YANG JD, HAINAUT P, GORES GJ, et al. A global view of hepatocellular carcinoma: Trends, risk, prevention and management[J]. Nat Rev Gastroenterol Hepatol, 2019, 16(10): 589-604. DOI: 10.1038/s41575-019-0186-y. [2] RAWLA P, SUNKARA T, MURALIDHARAN P, et al. Update in global trends and aetiology of hepatocellular carcinoma[J]. Contemp Oncol (Pozn), 2018, 22(3): 141-150. DOI: 10.5114/wo.2018.78941. [3] BLECHACZ B, MISHRA L. Hepatocellular carcinoma biology[J]. Recent Results Cancer Res, 2013, 190: 1-20. DOI: 10.1007/978-3-642-16037-0_1. [4] ZHANG J, ZHANG M, MA H, et al. Overexpression of glypican-3 is a predictor of poor prognosis in hepatocellular carcinoma: An updated meta-analysis[J]. Medicine (Baltimore), 2018, 97(24): e11130. DOI: 10.1097/MD.0000000000011130. [5] CHEN M, LI G, YAN J, et al. Reevaluation of glypican-3 as a serological marker for hepatocellular carcinoma[J]. Clin Chim Acta, 2013, 423: 105-111. DOI: 10.1016/j.cca.2013.04.026. [6] SUN B, HUANG Z, WANG B, et al. Significance of glypican-3 (GPC3) expression in hepatocellular cancer diagnosis[J]. Med Sci Monit, 2017, 23: 850-855. DOI: 10.12659/msm.899198. [7] BAUMHOER D, TORNILLO L, STADLMANN S, et al. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: A tissue microarray analysis of 4, 387 tissue samples[J]. Am J Clin Pathol, 2008, 129(6): 899-906. DOI: 10.1309/HCQWPWD50XHD2DW6. [8] WANG SK, ZYNGER DL, HES O, et al. Discovery and diagnostic value of a novel oncofetal protein: glypican 3[J]. Adv Anat Pathol, 2014, 21(6): 450-460. DOI: 10.1097/PAP.0000000000000043. [9] HARUYAMA Y, KATAOKA H. Glypican-3 is a prognostic factor and an immunotherapeutic target in hepatocellular carcinoma[J]. World J Gastroenterol, 2016, 22(1): 275-283. DOI: 10.3748/wjg.v22.i1.275. [10] DOLICKA D, SOBOLEWSKI C, CORREIA DE SOUSA M, et al. mRNA post-transcriptional regulation by AU-rich element-binding proteins in liver inflammation and cancer[J]. Int J Mol Sci, 2020, 21(18). DOI: 10.3390/ijms21186648. [11] LYKKE-ANDERSEN J, WAGNER E. Recruitment and activation of mRNA decay enzymes by two ARE-mediated decay activation domains in the proteins TTP and BRF-1[J]. Genes Dev, 2005, 19(3): 351-361. DOI: 10.1101/gad.1282305. [12] ZUCCONI BE, WILSON GM. Modulation of neoplastic gene regulatory pathways by the RNA-binding factor AUF1[J]. Front Biosci (Landmark Ed), 2011, 16: 2307-2325. DOI: 10.2741/3855. [13] MOORE AE, CHENETTE DM, LARKIN LC, et al. Physiological networks and disease functions of RNA-binding protein AUF1[J]. Wiley Interdiscip Rev RNA, 2014, 5(4): 549-564. DOI: 10.1002/wrna.1230. [14] GAO Y, WANG W, CAO J, et al. Upregulation of AUF1 is involved in the proliferation of esophageal squamous cell carcinoma through GCH1[J]. Int J Oncol, 2016, 49(5): 2001-2010. DOI: 10.3892/ijo.2016.3713. [15] DAI W, MU L, CUI Y, et al. Berberine promotes apoptosis of colorectal cancer via regulation of the long non-coding RNA (lncRNA) cancer susceptibility candidate 2 (CASC2)/AU-binding factor 1 (AUF1)/B-cell CLL/lymphoma 2 (Bcl-2) axis[J]. Med Sci Monit, 2019, 25: 730-738. DOI: 10.12659/MSM.912082. [16] ZHU XT, YUAN JH, ZHU TT, et al. Long noncoding RNA glypican 3 (GPC3) antisense transcript 1 promotes hepatocellular carcinoma progression via epigenetically activating GPC3[J]. FEBS J, 2016, 283(20): 3739-3754. DOI: 10.1111/febs.13839. [17] LI L, JIN R, ZHANG X, et al. Oncogenic activation of glypican-3 by c-Myc in human hepatocellular carcinoma[J]. Hepatology, 2012, 56(4): 1380-1390. DOI: 10.1002/hep.25891. [18] LUO JH, REN B, KERYANOV S, et al. Transcriptomic and genomic analysis of human hepatocellular carcinomas and hepatoblastomas[J]. Hepatology, 2006, 44(4): 1012-1024. DOI: 10.1002/hep.21328. [19] GAO Q, ZHU H, DONG L, et al. Integrated proteogenomic characterization of HBV-related hepatocellular carcinoma[J]. Cell, 2019, 179(2): 561-577. e22. DOI: 10.1016/j.cell.2019.08.052. 期刊类型引用(5)
1. 蔡纲,高庆娥,史元建. 富马酸替诺福韦酯和富马酸丙酚替诺福韦序贯联合聚乙二醇干扰素α-2b治疗慢性乙型肝炎患者的疗效比较. 黑龙江医药科学. 2024(01): 154-157 .  百度学术
百度学术2. 庄辉. 慢性乙型肝炎:从"只治"到"全治". 中华肝脏病杂志. 2024(05): 389-393 .  百度学术
百度学术3. 曾达武,朱月永. 非活动性HBsAg携带状态抗病毒治疗的利与弊. 临床内科杂志. 2023(12): 802-806 .  百度学术
百度学术4. 曾达武,朱月永. 非活动性乙型肝炎病毒表面抗原携带者的治疗. 国际流行病学传染病学杂志. 2022(04): 225-231 .  百度学术
百度学术5. 郑慧珂,孙昳,温瑾. 干扰素与核苷(酸)类似物对慢性乙型肝炎患者外周血调节性T细胞水平的影响. 中国实用医刊. 2021(04): 101-103 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 3927 KB)
PDF下载 ( 3927 KB)

 下载:
下载:


 百度学术
百度学术

