单核细胞/高密度脂蛋白胆固醇与非酒精性脂肪性肝病的相关性分析
DOI: 10.3969/j.issn.1001-5256.2021.05.031
Association between monocyte-to-high-density lipoprotein cholesterol ratio and nonalcoholic fatty liver disease
-
摘要:
目的 探讨单核细胞/高密度脂蛋白胆固醇(MHR)与非酒精性脂肪性肝病(NAFLD)的关系。 方法 选取2018年1月—2020年10月入住大连大学附属中山医院消化内科并经腹部CT诊断为NAFLD的208例患者作为NAFLD组,另选取同期210例健康体检人群设为对照组。所有受试者均行血常规、生化及腹部CT检查,计算血清MHR水平。另外,根据腹部CT的影像学结果,将NAFLD患者分为轻度NAFLD组(n=148)及中重度NAFLD组(n=60),观察不同程度NAFLD患者与对照组WBC及MHR等指标的差异。正态分布计量资料两组间比较采用独立样本t检验,多组间比较采用单因素方差分析。偏态分布计量资料两组间比较采用Mann-Whitney U秩和检验,多组间采用Kruskal-Wallis H检验。计数资料两组间比较采用四格表χ2检验,3组间比较采用R×C表χ2检验。MHR与各代谢指标及NAFLD严重程度间的相关性采用Spearman相关分析。绘制受试者工作特征曲线评估MHR对NAFLD的诊断价值。 结果 与对照组相比,NAFLD组中的体质量(t=-10.573, P < 0.001)、BMI(t=-13.112, P < 0.001)、吸烟史(χ2=14.667, P < 0.001)、WBC(t=-7.359, P < 0.001)、单核细胞(Z=-9.932, P < 0.001)、LDL-C(t=-3.394, P=0.001)、TG(Z=-11.737, P < 0.001)、CHO(t=-2.985, P=0.003)、空腹血糖(Z=-7.827, P < 0.001)、ALT(Z=-12.583, P < 0.001)、AST(Z=-9.514, P < 0.001) 水平均增加,而血清HDL-C(t=10.440, P < 0.001)水平下降;另外,MHR水平存在性别差异,男性明显高于女性,差异有统计学意义(P < 0.001)。与对照组及轻度NAFLD组相比,血清MHR水平在中重度NAFLD组中显著升高,差异有统计学意义(P值均 < 0.001)。相关分析结果提示血清MHR水平与HDL-C呈负相关(r=-0.565, P < 0.001),与吸烟史、体质量、BMI、WBC、单核细胞、TG、空腹血糖、ALT、AST呈正相关(r值分别为0.449、0.482、0.430、0.478、0.892、0.333、0.157、0.386、0.281,P值均 < 0.01)。同时,MHR水平与NAFLD严重程度呈正相关(r=0.629,P < 0.001)。ROC曲线表明MHR曲线下面积为0.846(95%CI:0.810~0.882,P < 0.001),敏感度和特异度分别为77.9%和74.3%。 结论 血清MHR水平与NAFLD相关,可作为评价NAFLD病情进展的一种预测指标。 Abstract:Objective To investigate the association between monocyte-to-high-density lipoprotein cholesterol ratio (MHR) and nonalcoholic fatty liver disease (NAFLD). Methods A total of 208 patients who were admitted to Department of Gastroenterology, Zhongshan Hospital Affiliated to Dalian University, from January 2018 to October 2020 and were diagnosed with NAFLD by abdominal CT were enrolled as NAFLD group, and 210 healthy individuals were enrolled as control group. All subjects underwent routine blood test, biochemical examination, and abdominal CT examination, and serum MHR was calculated. In addition, according to abdominal CT findings, the patients with NAFLD were divided into mild NAFLD group with 148 patients and moderate-to-severe NAFLD group with 60 patients, and the variables such as white blood cell count (WBC) and MHR were compared between the three groups. The independent samples t-test was used for comparison of normally distributed continuous data between two groups, and a one-way analysis of variance was used for comparison between multiple groups; the Mann-Whitney U test was used for comparison of data with skewed distribution between two groups, and the Kruskal-Wallis H test was used for comparison between multiple groups. The fourfold table chi-square test was used for comparison of categorical data between two groups, and the R×C table chi-square test was used for comparison between three groups. A Spearman correlation analysis was used to investigate the correlation of MHR with metabolic markers and the severity of NAFLD. The receiver operating characteristic (ROC) curve was used to evaluate the diagnostic value of MHR in predicting NAFLD. Results Compared with the control group, the NAFLD group had significant increases in body weight (t=-10.573, P < 0.001), body mass index (BMI) (t=-13.112, P < 0.001), smoking history (Z=14.667, P < 0.001), WBC (t=-7.359, P < 0.001), monocytes (Z=-9.932, P < 0.001), low-density lipoprotein cholesterol (t=-3.394, P=0.001), triglyceride (TG) (Z=-11.737, P < 0.001), cholesterol (t=-2.985, P=0.003), fasting blood glucose (FBG) (Z=-7.827, P < 0.001), alanine aminotransferase (ALT) (Z=-12.583, P < 0.001), and aspartate aminotransferase (AST) (Z=-9.514, P < 0.001) and a significant reduction in serum high-density lipoprotein cholesterol (HDL-C) (t=10.440, P < 0.001); in addition, MHR level had gender differences, and male patients had a significantly higher level than female patients (P < 0.001). Compared with the control group and the mild NAFLD group, the moderate-to-severe NAFLD group had a significant increase in serum MHR level (P < 0.001). The correlation analysis showed that serum MHR level was negatively correlated with HDL-C (r=-0.565, P < 0.001) and were positively correlated with smoking history, body weight, BMI, WBC, monocytes, TG, FBG, ALT, and AST (r=0.449, 0.482, 0.430, 0.478, 0.892, 0.333, 0.157, 0.386, and 0.281, all P < 0.01). At the same time, MHR level was positively correlated with the severity of NAFLD (r=0.629, P < 0.001). The ROC curve showed that MHR had an area under the ROC curve of 0.846 (95% confidence interval: 0.810-0.882, P < 0.001), with a sensitivity of 77.9% and a specificity of 74.3%. Conclusion Serum MHR level is associated with NAFLD and can be used as a predictive index for evaluating the progression of NAFLD. -
Key words:
- Non-alcoholic Fatty Liver Disease /
- Monocytes /
- Cholesterol, HDL
-
在过去的二十年中,非酒精性脂肪性肝病(NAFLD)已经从一种相对无人问津的疾病迅速演变成全球最常见的慢性肝病[1],可能会进展为肝纤维化、肝硬化,甚至肝癌,还会增加罹患心血管疾病、糖尿病等慢性病的风险。近年发现,单核细胞/高密度脂蛋白胆固醇(MHR)可作为一种新型炎性指标[2],因与其他炎症因子相比,获取方便,重复性好,价格低廉,备受学者喜爱。有研究[3-6]证实,MHR与糖尿病、高血压、多囊卵巢综合征、代谢综合征等密切相关,并与C反应蛋白呈正相关。NAFLD作为代谢综合征在肝脏的表现,目前尚未报道相关研究。本课题旨在探讨血清MHR水平与NAFLD的关系,以为临床诊断及评估NAFLD进展提供一定的理论参考。
1. 资料与方法
1.1 研究对象
收集2018年1月—2020年10月入住本院消化内科且经腹部CT诊断为NAFLD的患者的临床资料,诊断标准均符合《非酒精性脂肪性肝病防治指南(2018年更新版)》[7]。排除药物性及病毒性肝病、恶性肿瘤及心肾功能不全者;患有血液性及感染性疾病等。另选取同期健康体检人群作为对照组。
1.2 一般资料采集
受试者均进行人体测量及个人嗜好采集,记录身高、体质量、年龄、吸烟等信息,并计算BMI。
1.3 生化指标检测
留取所有患者晨起或空腹12 h以上的肘静脉血3~5 ml,予离心机(TDZ5-WS)以3500 r/min离心5 min,于全自动生化分析仪检测实验性参数,包括WBC、单核细胞计数、TG、CHO、HDL、LDL、空腹血糖(FBG)、ALT、AST。计算MHR=单核细胞/HDL-C。
1.4 NAFLD及严重程度的诊断
受试者均行全腹CT检查,由同一名具有丰富经验的技师行影像学解读。CT诊断脂肪肝的依据为肝脏密度普遍降低,肝/脾CT值之比 < 1.0。其中,肝/脾CT比值< 1.0但>0.7者为轻度,≤0.7但>0.5者为中度,≤0.5者为重度脂肪肝[8]。
1.5 伦理学审查
本研究通过大连大学附属中山医院伦理委员会批准,批号:2020038,均获得受试者同意。
1.6 统计学方法
采用SPSS 20.0和Graphpad prism 8.0.2软件处理数据及绘图。正态分布计量资料以x±s表示,两组间比较采用独立样本t检验,多组间比较采用单因素方差分析。偏态分布计量资料以M(P25~P75)表示,两组间比较采用Mann-Whitney U秩和检验,多组间采用Kruskal-Wallis H秩和检验。计数资料两组间比较采用四格表χ2检验,3组间比较采用R×C表χ2检验。MHR与各代谢指标及NAFLD严重程度间的相关性采用Spearman相关分析。绘制受试者工作特征曲线(ROC曲线)分析MHR对NAFLD的诊断价值。P < 0.05为差异具有统计学意义。
2. 结果
2.1 一般情况
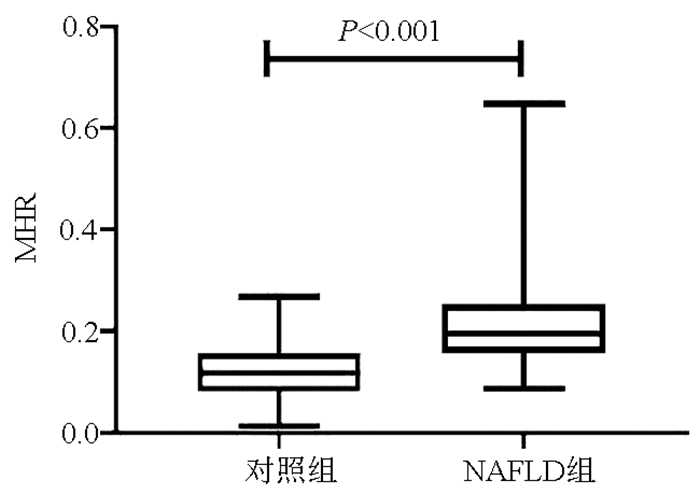
共纳入NAFLD患者208例,健康体检者210例为对照组。与对照组相比,NAFLD组中的吸烟史比例、体质量、BMI、WBC、单核细胞、LDL-C、TG、CHO、FBG、ALT、AST水平均升高,HDL-C水平降低,差异均有统计学意义(P值均 < 0.05),两组间性别、年龄及身高相比,差异均无统计学意义(P值均>0.05)。NAFLD组中血清MHR水平显著高于对照组(P < 0.001)(表 1,图 1)。
表 1 对照组与NAFLD组患者一般情况的比较指标 对照组(n=210) NAFLD组(n=208) 统计值 P值 男/女(例) 95/115 114/94 χ2=3.828 >0.050 年龄(岁) 52(44~61) 55(43~62) Z=-1.066 0.286 吸烟[例(%)] 30(14.29) 62(29.81) χ2=14.667 < 0.001 身高(cm) 167(162~172) 170(162~175) Z=-1.891 0.059 体质量(kg) 64.64±10.83 77.80±14.35 t=-10.573 < 0.001 BMI(kg/m2) 22.88±2.87 27.03±3.56 t=-13.112 < 0.001 WBC(×109/L) 5.11±1.14 6.00±1.31 t=-7.359 < 0.001 单核细胞(×109/L) 0.16(0.13~0.20) 0.23(0.20~0.30) Z=-9.932 < 0.001 HDL-C(mmol/L) 1.45±0.30 1.18±0.22 t=10.440 < 0.001 MHR 0.12(0.08~0.16) 0.20(0.16~0.25) Z=-12.233 < 0.001 LDL-C(mmol/L) 2.88±0.68 3.13±0.78 t=-3.394 0.001 TG(mmol/L) 1.15(0.87~1.44) 1.92(1.49~2.86) Z=-11.737 < 0.001 CHO(mmol/L) 4.99±0.90 5.28±1.02 t=-2.985 0.003 FBG(mmol/L) 4.88(4.55~5.21) 5.34(4.89~6.06) Z=-7.827 < 0.001 ALT(U/L) 16.0(12.0~24.0) 36.0(25.0~54.5) Z=-12.583 < 0.001 AST(U/L) 18.00(15.00~21.00) 24.00(18.00~30.75) Z=-9.514 < 0.001 2.2 对照组与不同程度NAFLD患者一般情况的比较
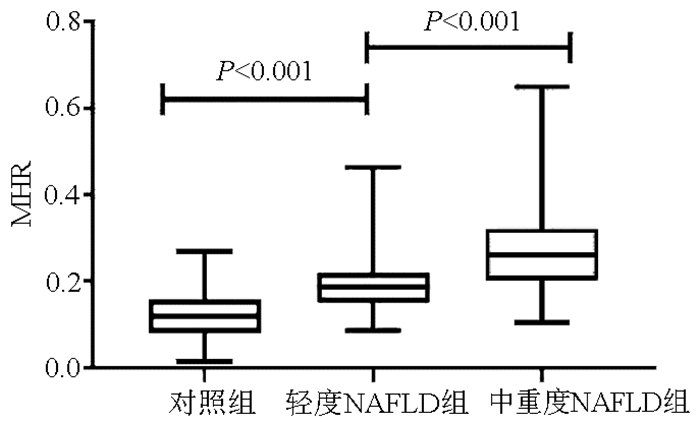
不同程度NAFLD患者与对照组比较,性别、吸烟史、体质量、BMI、WBC、单核细胞、HDL-C、MHR、LDL-C、TG、CHO、FBG、ALT、AST等指标均有差异(P值均 < 0.05)。其中中重度NAFLD组血清MHR水平高于轻度NAFLD组及对照组,差异均有统计学意义(P值均 < 0.001)(表 2,图 2)。
表 2 对照组与不同程度NAFLD患者一般情况的比较指标 对照组(n=210) 轻度NAFLD组(n=148) 中重度NAFLD组(n=60) 统计值 P值 男/女(例) 95/115 75/73 39/21 χ2=7.332 0.026 年龄(岁) 51.40±11.78 53.61±11.50 50.43±13.32 F=2.141 0.119 吸烟[例(%)] 30(14.29) 36(24.32) 26(43.33) H=23.654 < 0.001 身高(cm) 167.0(162.0~172.0) 169.0(161.3~175.0) 170.5(162.0~177.0) H=5.292 0.071 体质量(kg) 64.64±10.83 76.67±12.84 80.60±17.36 F=58.503 < 0.001 BMI(kg/m2) 22.88±2.87 26.81±3.18 27.58±4.35 F=129.920 < 0.001 WBC(×109/L) 5.11±1.14 5.79±1.17 6.51±1.49 F=54.130 < 0.001 单核细胞(×109/L) 0.16(0.13~0.20) 0.21(0.19~0.26) 0.30(0.22~0.35) H=115.697 < 0.001 HDL-C(mmol/L) 1.45±0.30 1.21±0.23 1.13±0.19 F=88.936 < 0.001 MHR 0.12(0.08~0.16) 0.19(0.15~0.22) 0.26(0.20~0.32) H=167.607 < 0.001 LDL-C(mmol/L) 2.88±0.68 3.18±0.79 2.99±0.75 F=7.180 0.001 TG(mmol/L) 1.15(0.87~1.44) 1.91(1.53~2.82) 1.96(1.45~3.11) H=137.865 < 0.001 CHO(mmol/L) 4.99±0.90 5.33±0.99 5.14±1.11 F=5.278 0.005 FBG(mmol/L) 4.88(4.55~5.21) 5.34(4.87~6.05) 5.35(4.94~6.06) H=61.521 < 0.001 ALT(U/L) 16.00(12.00~24.00) 32.00(21.78~50.00) 46.00(32.35~60.75) H=167.151 < 0.001 AST(U/L) 18.00(15.00~21.00) 23.00(17.25~29.00) 24.50(21.00~33.75) H=95.963 < 0.001 2.3 血清MHR水平在性别之间的比较
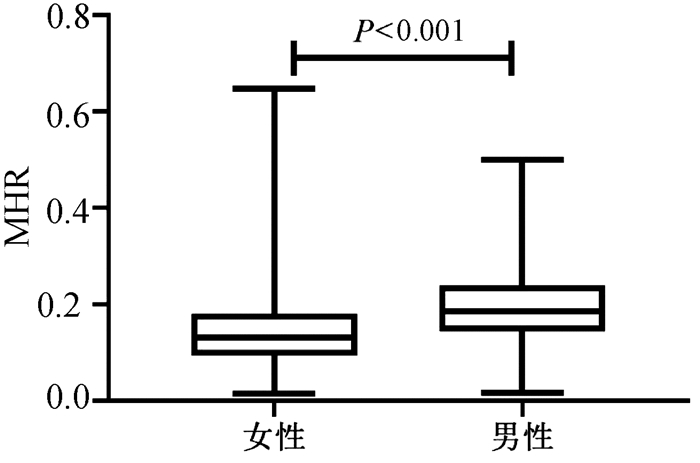
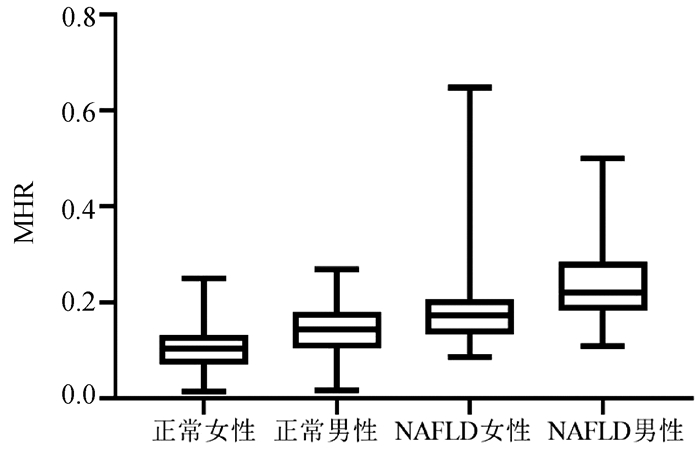
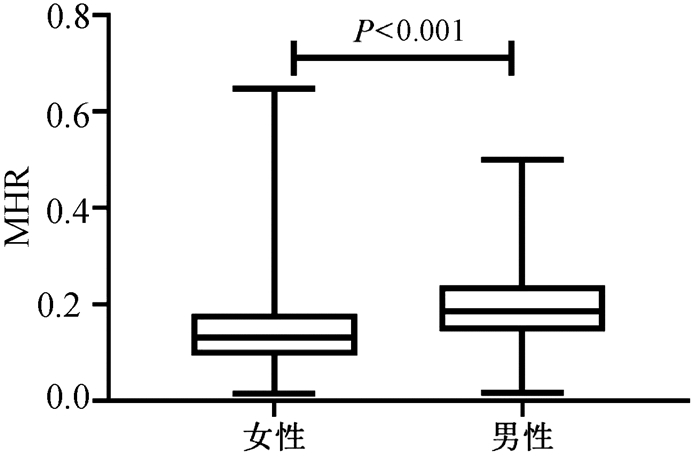
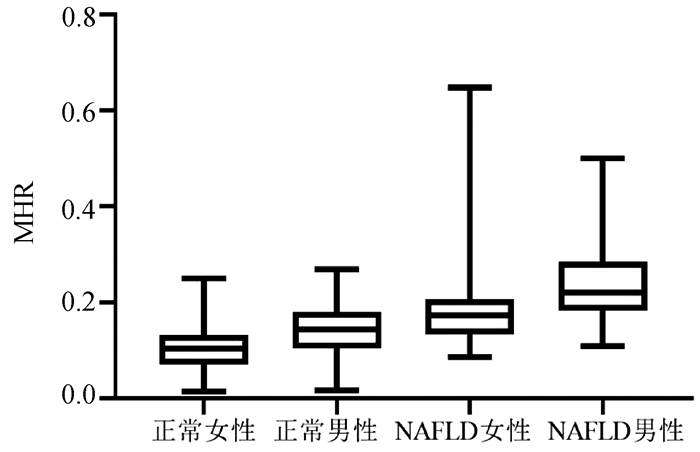
按性别分组,结果表明男性的血清MHR水平[0.186(0.143~0.239)]较女性[0.131(0.094~0.180)]明显增加(P < 0.001)(图 3)。血清MHR在正常女性、正常男性、NAFLD女性、NAFLD男性的水平分别为[0.10(0.07~0.13)、0.14(0.10~0.18)、0.17(0.13~0.21)、0.22(0.18~0.28)],差异有统计学意义(H=192.35, P < 0.001)(图 4)。
2.4 血清MHR水平与各变量的相关性分析
相关分析结果显示: MHR与HDL-C呈负相关,与吸烟史、体质量、BMI、WBC、单核细胞、TG、FBG、ALT、AST呈正相关,同时MHR水平与NAFLD的严重程度亦呈正相关(P值均 < 0.05)(表 3)。
表 3 血清MHR水平与各变量的相关性变量 r值 P值 年龄 -0.094 0.054 吸烟史 0.449 < 0.001 体质量 0.482 < 0.001 BMI 0.430 < 0.001 WBC 0.478 < 0.001 单核细胞 0.892 < 0.001 HDL-C -0.565 < 0.001 LDL-C 0.013 0.781 TG 0.333 < 0.001 CHO -0.071 0.145 FBG 0.157 0.001 ALT 0.386 < 0.001 AST 0.281 < 0.001 NAFLD严重性 0.629 < 0.001 2.5 MHR诊断NAFLD的ROC曲线
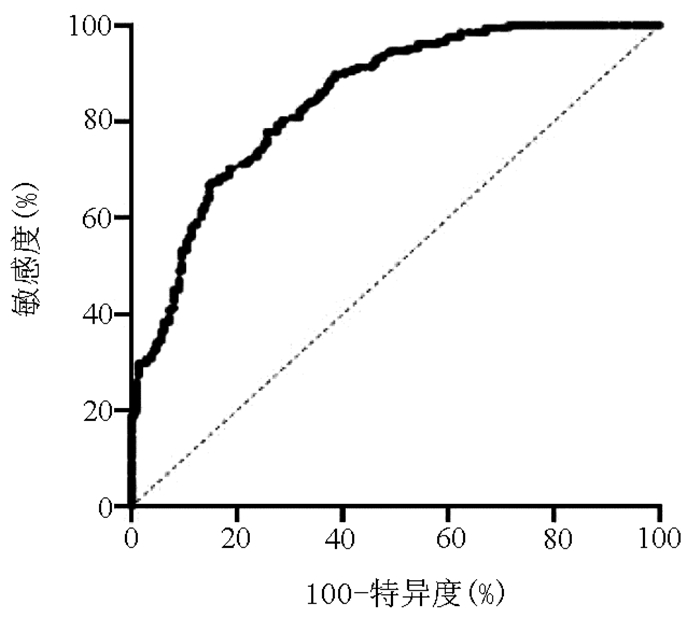
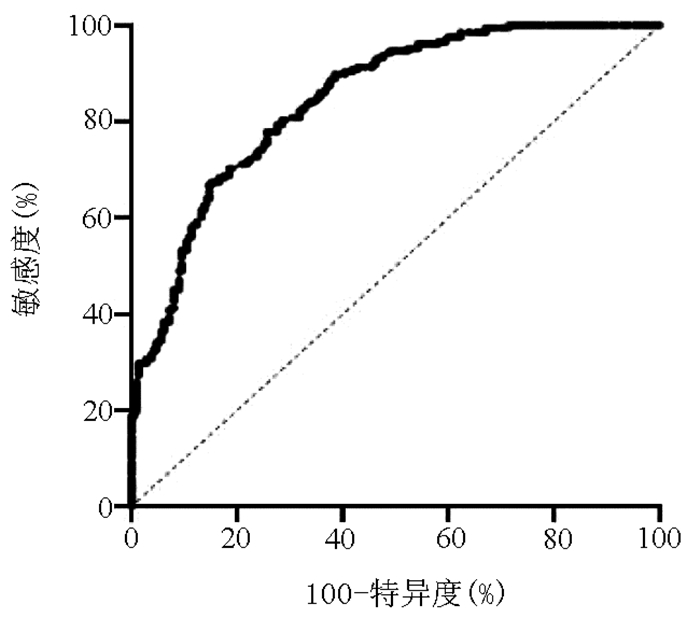
探索MHR对NAFLD的潜在诊断价值,结果示MHR的ROC曲线下面积为0.846(95%CI:0.810~0.882,P < 0.001)。当MHR的最佳截断值为0.522时,诊断NAFLD的敏感度为77.9%,特异度为74.3%(图 5)。
3. 讨论
NAFLD已成为西方国家肝移植的第二大原因,预计未来3~5年位居首位[9],其病因错综复杂,至今尚未定论,但慢性炎症[10]在NAFLD的机制中已得到充分认同。我国王树康等[11]学者在一项大型纵向队列研究中发现,排除多种混杂变量后,外周血WBC水平越高,发生NAFLD的风险随之增加。单核细胞肩负机体防御和调节组织稳态的职责,被Zhang等[12]发现在NAFLD患者中显著增加,且中间单核细胞(CD14++CD16+)可作为NAFLD的独立危险因素。本研究也发现NAFLD患者中的WBC及单核细胞显著高于对照组,在不同程度的NAFLD患者中也显示较大差异,与既往研究结论一致,再次证实炎症与NAFLD密不可分。
MHR作为两项常规生化指标的比值,被当代视为新生炎症标志物,大量研究[3-5]证实,MHR可安全、有效预测疾病的进展及预后。我国一项心血管健康数据[13]显示,MHR水平与缺血性卒中概率之间存在线性关系,且MHR最高四分位数的患者与最低组相比发生缺血性卒中的风险高出1.6倍,同时Li等[14]发现在阻塞性睡眠呼吸暂停中MHR较健康人群增加明显,并与呼吸暂停低通气指数呈正相关。
在本研究中,与对照组相比,NAFLD患者的血清MHR水平显著增加,并随着NAFLD病情的恶化而随之上升。据笔者所知,这是首次开展MHR与NAFLD的相关研究。其次,相关分析显示,血清MHR水平与HDL-C呈负相关,与吸烟史、WBC、TG、FBG、ALT、AST及NAFLD严重程度呈正相关,表明MHR涉及炎症、糖脂代谢等多个方面,是一个综合性指标, 与Jialal等[15]、YIlmaz等[2]结论无明显差异。再次,以性别分组,血清MHR水平存在性别差异,即男性高于女性,在对照组及NAFLD组内比较也提示显著差异,可能是由于男性多合并吸烟等不良嗜好,承担的社会压力较大,性激素对体脂分布的差异等造成的。最后,绘制ROC曲线分析MHR对NAFLD的诊断效能,结果表明MHR的ROC曲线下面积为0.846,敏感度为77.9%,特异度为74.3%,故一定程度上可用来区分对照组及NAFLD组。但MHR与NAFLD具体的机制仍未知晓,可能有以下可能:首先是慢性炎症,众所周知,全身循环系统的大多数促炎和氧化细胞因子来源于单核细胞[16],而脂质蓄积是NAFLD的基本特征,可导致肝细胞损伤,随之促炎细胞因子大量释放,包括外周的单核细胞及其衍生的浸润型巨噬细胞,加速NAFLD向非酒精性脂肪性肝炎的演变进程[17]。同时,胆汁酸双受体激活剂(INT-767)可通过介导单核细胞免疫表型的转变,提高Ly6clow单核细胞的含量,从而减轻NAFLD的炎症反应[18]。其次是胰岛素抵抗,它是NAFLD作用机制的首发环节。Glass等[19]研究发现机体脂肪组织胰岛素抵抗主要起源于单核细胞分化的M1促炎性巨噬细胞,慢性炎症与胰岛素抵抗密切相关。
本研究的局限性:(1)回顾性研究,未能证实NAFLD患者中MHR与其他炎性指标的相关性;(2)无法确定MHR与其他有统计学意义变量之间的因果关系。
总而言之,本研究首次发现,MHR水平在NAFLD患者中呈现异常,并与NAFLD的进展密切相关。在今后NAFLD的实践工作中,MHR有望作为一种价格低廉、易于测量及处理的指标用来预测NAFLD,未来还需要更多大样本研究验证。
-
表 1 对照组与NAFLD组患者一般情况的比较
指标 对照组(n=210) NAFLD组(n=208) 统计值 P值 男/女(例) 95/115 114/94 χ2=3.828 >0.050 年龄(岁) 52(44~61) 55(43~62) Z=-1.066 0.286 吸烟[例(%)] 30(14.29) 62(29.81) χ2=14.667 < 0.001 身高(cm) 167(162~172) 170(162~175) Z=-1.891 0.059 体质量(kg) 64.64±10.83 77.80±14.35 t=-10.573 < 0.001 BMI(kg/m2) 22.88±2.87 27.03±3.56 t=-13.112 < 0.001 WBC(×109/L) 5.11±1.14 6.00±1.31 t=-7.359 < 0.001 单核细胞(×109/L) 0.16(0.13~0.20) 0.23(0.20~0.30) Z=-9.932 < 0.001 HDL-C(mmol/L) 1.45±0.30 1.18±0.22 t=10.440 < 0.001 MHR 0.12(0.08~0.16) 0.20(0.16~0.25) Z=-12.233 < 0.001 LDL-C(mmol/L) 2.88±0.68 3.13±0.78 t=-3.394 0.001 TG(mmol/L) 1.15(0.87~1.44) 1.92(1.49~2.86) Z=-11.737 < 0.001 CHO(mmol/L) 4.99±0.90 5.28±1.02 t=-2.985 0.003 FBG(mmol/L) 4.88(4.55~5.21) 5.34(4.89~6.06) Z=-7.827 < 0.001 ALT(U/L) 16.0(12.0~24.0) 36.0(25.0~54.5) Z=-12.583 < 0.001 AST(U/L) 18.00(15.00~21.00) 24.00(18.00~30.75) Z=-9.514 < 0.001 表 2 对照组与不同程度NAFLD患者一般情况的比较
指标 对照组(n=210) 轻度NAFLD组(n=148) 中重度NAFLD组(n=60) 统计值 P值 男/女(例) 95/115 75/73 39/21 χ2=7.332 0.026 年龄(岁) 51.40±11.78 53.61±11.50 50.43±13.32 F=2.141 0.119 吸烟[例(%)] 30(14.29) 36(24.32) 26(43.33) H=23.654 < 0.001 身高(cm) 167.0(162.0~172.0) 169.0(161.3~175.0) 170.5(162.0~177.0) H=5.292 0.071 体质量(kg) 64.64±10.83 76.67±12.84 80.60±17.36 F=58.503 < 0.001 BMI(kg/m2) 22.88±2.87 26.81±3.18 27.58±4.35 F=129.920 < 0.001 WBC(×109/L) 5.11±1.14 5.79±1.17 6.51±1.49 F=54.130 < 0.001 单核细胞(×109/L) 0.16(0.13~0.20) 0.21(0.19~0.26) 0.30(0.22~0.35) H=115.697 < 0.001 HDL-C(mmol/L) 1.45±0.30 1.21±0.23 1.13±0.19 F=88.936 < 0.001 MHR 0.12(0.08~0.16) 0.19(0.15~0.22) 0.26(0.20~0.32) H=167.607 < 0.001 LDL-C(mmol/L) 2.88±0.68 3.18±0.79 2.99±0.75 F=7.180 0.001 TG(mmol/L) 1.15(0.87~1.44) 1.91(1.53~2.82) 1.96(1.45~3.11) H=137.865 < 0.001 CHO(mmol/L) 4.99±0.90 5.33±0.99 5.14±1.11 F=5.278 0.005 FBG(mmol/L) 4.88(4.55~5.21) 5.34(4.87~6.05) 5.35(4.94~6.06) H=61.521 < 0.001 ALT(U/L) 16.00(12.00~24.00) 32.00(21.78~50.00) 46.00(32.35~60.75) H=167.151 < 0.001 AST(U/L) 18.00(15.00~21.00) 23.00(17.25~29.00) 24.50(21.00~33.75) H=95.963 < 0.001 表 3 血清MHR水平与各变量的相关性
变量 r值 P值 年龄 -0.094 0.054 吸烟史 0.449 < 0.001 体质量 0.482 < 0.001 BMI 0.430 < 0.001 WBC 0.478 < 0.001 单核细胞 0.892 < 0.001 HDL-C -0.565 < 0.001 LDL-C 0.013 0.781 TG 0.333 < 0.001 CHO -0.071 0.145 FBG 0.157 0.001 ALT 0.386 < 0.001 AST 0.281 < 0.001 NAFLD严重性 0.629 < 0.001 -
[1] ESLAM M, SANYAL AJ, GEORGE J, et al. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease[J]. Gastroenterology, 2020, 158(7): 1999-2014. e1. DOI: 10.1053/j.gastro.2019.11.312. [2] YILMAZ M, KAYANÇIÇEK H. A new inflammatory marker: Elevated monocyte to HDL cholesterol ratio associated with smoking[J]. J Clin Med, 2018, 7(4): 76. DOI: 10.3390/jcm7040076. [3] KARATAS A, TURKMEN E, ERDEM E, et al. Monocyte to high-density lipoprotein cholesterol ratio in patients with diabetes mellitus and diabetic nephropathy[J]. Biomark Med, 2018, 12(9): 953-959. DOI: 10.2217/bmm-2018-0048. [4] KAPLAN IG, KAPLAN M, ABACIOGLU OO, et al. Monocyte/HDL ratio predicts hypertensive complications[J]. Bratisl Lek Listy, 2020, 121(2): 133-136. DOI: 10.4149/BLL_2020_018. [5] USTA A, AVCI E, BULBUL CB, et al. The monocyte counts to hdlcholesterol ratio in obese and lean patients with polycystic ovary syndrome[J]. Reprod Biol Endocrinol, 2018, 16 (1): 34. DOI: 10.1186/s12958-018-0351-0. [6] VAHIT D, AKBOGA MK, SAMET Y, et al. Assessment of monocyte to high density lipoprotein cholesterol ratio and lymphocyte-to-monocyte ratio in patients with metabolic syndrome[J]. Biomark Med, 2017, 11(7): 535-540. DOI: 10.2217/bmm-2016-0380. [7] Chinese Medical Association Liver Diseases Branch Fatty Liver and Alcoholic Liver Disease Group, Guidelines for the prevention and treatment of nonalcoholic fatty liver disease (updated version 2018) [J]. J Clin Hepatol, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007.中华医学会肝病学分会脂肪肝和酒精性肝病学组. 非酒精性脂肪性肝病防治指南(2018年更新版)[J]. 临床肝胆病杂志, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007. [8] ALSWAT KA, FALLATAH HI, AL-JUDAIBI B, et al. Position statement on the diagnosis and management of non-alcoholic fatty liver disease[J]. Saudi Med J, 2019, 40(6): 531-540. DOI: 10.15537/smj.2019.6.23980. [9] MALIAKKAL BJ. Pathogenesis of non-alcoholic fatty liver disease and implications on cardiovascular outcomes in liver transplantation[J]. Transl Gastroenterol Hepatol, 2020, 5: 36. DOI: 10.21037/tgh.2019.12.02. [10] SHAO M, YE Z, QIN Y, et al. Abnormal metabolic processes involved in the pathogenesis of non-alcoholic fatty liver disease (Review)[J]. Exp Ther Med, 2020, 20(5): 26. DOI: 10.3892/etm.2020.9154. [11] WANG S, ZHANG C, ZHANG G, et al. Association between white blood cell count and non-alcoholic fatty liver disease in urban Han Chinese: A prospective cohort study[J]. BMJ Open, 2016, 6(6): e010342. DOI: 10.1136/bmjopen-2015-010342. [12] ZHANG J, CHEN W, FANG L, et al. Increased intermediate monocyte fraction in peripheral blood is associated with nonalcoholic fatty liver disease[J]. Wien Klin Wochenschr, 2018, 130(11-12): 390-397. DOI: 10.1007/s00508-018-1348-6. [13] WANG HY, SHI WR, YI X, et al. Assessing the performance of monocyte to high-density lipoprotein ratio for predicting ischemic stroke: Insights from a population-based Chinese cohort[J]. Lipids Health Dis, 2019, 18(1): 127. DOI: 10.1186/s12944-019-1076-6. [14] LI N, REN L, WANG JH, et al. Relationship between monocyte to HDL cholesterol ratio and concomitant cardiovascular disease in Chinese Han patients with obstructive sleep apnea[J]. Cardiovasc Diagn Ther, 2019, 9(4): 362-370. DOI: 10.21037/cdt.2019.08.02. [15] JIALAL I, JIALAL G, ADAMS-HUET B, et al. Neutrophil and monocyte ratios to high-density lipoprotein-cholesterol and adiponectin as biomarkers of nascent metabolic syndrome[J]. Horm Mol Biol Clin Investig, 2020, 41(2). DOI: 10.1515/hmbci-2019-0070. [16] ANCUTA P, WANG J, GABUZDA D. CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells[J]. J Leukoc Biol, 2006, 80(5): 1156-1164. DOI: 10.1189/jlb.0206125. [17] CHA JY, KIM DH, CHUN KH. The role of hepatic macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis[J]. Lab Anim Res, 2018, 34(4): 133-139. DOI: 10.5625/lar.2018.34.4.133. [18] MCMAHAN RH, WANG XX, CHENG LL, et al. Bile acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic fatty liver disease[J]. J Biol Chem, 2013, 288(17): 11761-11770. DOI: 10.1074/jbc.M112.446575. [19] GLASS CK, OLEFSKY JM. Inflammation and lipid signaling in the etiology of insulin resistance[J]. Cell Metab, 2012, 15(5): 635-645. DOI: 10.1016/j.cmet.2012.04.001. 期刊类型引用(4)
1. 于萍,孟洁,刘晓玲,覃洋,王嘉,秦杰. TG/HDL-C、GGT在非酒精性脂肪肝病患者中的水平变化及临床价值. 中国老年学杂志. 2025(01): 72-75 .  百度学术
百度学术2. 陈海军,刘翟,史越,李雨泽,郭洪霞,鲍金华,许超蕊,张堃. 基于随机森林模型内脏脂肪等级相关指标分析. 中华健康管理学杂志. 2023(01): 41-46 .  百度学术
百度学术3. 宋凡凡,杨彩彩,赵旭敏,陈瑛,李宇,陈宇,贾爱华. 身体测量指标及代谢指标对2型糖尿病患者合并非酒精性脂肪肝的诊断价值分析. 国际医药卫生导报. 2023(01): 78-82 .  百度学术
百度学术4. 张尊祺,章福彬,史靖. 单核细胞计数/高密度脂蛋白胆固醇比值与急性胰腺炎的相关性研究. 肝胆外科杂志. 2023(06): 440-443 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 2065 KB)
PDF下载 ( 2065 KB)

 下载:
下载:





 下载:
下载:
 百度学术
百度学术