预后营养指数对肝泡型包虫病患者预后的预测价值
DOI: 10.3969/j.issn.1001-5256.2021.05.033
Value of prognostic nutritional index in predicting the prognosis of patients with hepatic alveolar echinococcosis
-
摘要:
目的 探讨预后营养指数(PNI)与肝泡型包虫病患者预后的关系。 方法 回顾性分析2015年1月—2018年12月青海大学附属医院肝胆胰外科收治的行肝泡型包虫病手术治疗的242例患者的临床资料。收集患者术前1周血常规及肝功能结果,计算PNI数值。通过X-Tile软件寻找PNI的临界值,并根据该临界值将患者分为高PNI组(n=216)和低PNI组(n=26)。计数资料两组两间比较采用χ2检验;Kaplan-Meier法绘制生存曲线,组间差异比较采用log-rank检验;采用Cox回归模型进行单因素和多因素分析,并计算风险比(HR)及对应的95%CI。 结果 PNI与治疗方式、术中出血量、包虫分期、病灶数、Child-Pugh分级、AST、TBil、Alb、ALP、PT、淋巴细胞计数均有关(P值均 < 0.05)。低PNI组与高PNI组患者术后1、3、5年生存率分别为92.1%、30.5%、20.3%和95.7%、79.5%、56.9%;低PNI组平均生存时间为33.783个月(95%CI:26.450~51.355),高PNI组平均生存时间为51.355个月(95%CI:49.044~53.666),两组比较差异有统计学意义(χ2=24.626,P < 0.001)。单因素Cox回归结果显示,PNI、手术方式、术中出血量、包虫分期、Child-Pugh分级、并发症、ALP、PT、中性粒细胞、血小板均与肝泡型包虫病患者预后有关(P值均 < 0.05);多因素Cox回归结果显示,治疗方式(HR=1.461,95%CI:1.132~1.890,P=0.004)、术中出血量(HR=6.078,95%CI:3.412~10.826,P < 0.001)、PNI(HR=0.367,95%CI:0.192~0.701,P=0.002)、并发症(HR=2.007,95%CI:1.204~3.346,P=0.008)、中性粒细胞(HR=2.772,95%CI:1.304~5.891,P=0.008)均是影响肝泡型包虫病患者预后的独立危险因素。 结论 PNI可以作为预测肝泡型包虫病患者术后的预后指标,术前外周血PNI越高,患者预后越好。 Abstract:Objective To investigate the value of prognostic nutritional index (PNI) in predicting the prognosis of patients with hepatic alveolar echinococcosis. Methods A retrospective analysis was performed for the clinical data of 242 patients who were admitted to Department of Hepatopancreatobiliary Surgery, Qinghai University Affiliated Hospital, from January 2015 to December 2018 and underwent surgical treatment of hepatic alveolar echinococcosis. The results of routine blood test and liver function were collected at 1 week before surgery, and PNI was calculated. X-Tile software was used to determine the cut-off value of PNI, and according to this cut-off value, the patients were divided into high PNI group with 216 patients and low PNI group with 26 patients. The chi-square test was used for comparison of categorical data between groups; the Kaplan-Meier method was used to plot survival curves, and the log-rank test was used for comparison between groups; the Cox regression model was used to perform univariate and multivariate analyses, and hazard ratio (HR) and corresponding 95% confidence interval (CI) were calculated. Results PNI was associated with treatment modality, intraoperative blood loss, stage of echinococcosis, number of lesions, Child-Pugh class, aspartate aminotransferase, total bilirubin, albumin, alkaline phosphatase (ALP), prothrombin time (PT), and lymphocyte count (all P < 0.05). The low PNI group had 1-, 3-, and 5-year survival rates of 92.1%, 30.5%, and 20.3%, respectively, after surgery, while the high PNI group had 1-, 3-, and 5-year survival rates of 95.7%, 79.5%, and 56.9%, respectively. The mean survival time was 33.783 (95% CI: 26.450-51.355) months in the low PNI group and 51.355 (95% CI: 49.044-53.666) months in the high PNI group, and there was a significant difference between the two groups (χ2=24.626, P < 0.001). The univariate Cox regression analysis showed that PNI, surgical procedure, intraoperative blood loss, stage of echinococcosis, Child-Pugh class, complication, ALP, PT, neutrophils, and platelets were associated with the prognosis of patients with hepatic alveolar echinococcosis (all P < 0.05), and the multivariate Cox regression analysis showed that treatment modality (HR=1.461, 95%CI: 1.132-1.890, P=0.004), intraoperative blood loss (HR=6.078, 95%CI: 3.412-10.826, P < 0.001), PNI(HR=0.367, 95%CI: 0.192-0.701, P=0.002), complications (HR=2.007, 95%CI: 1.204-3.346, P=0.008), and neutrophils (HR=2.772, 95%CI: 1.304-5.891, P=0.008) were independent risk factors for the prognosis of patients with hepatic alveolar echinococcosis. Conclusion PNI can be used to predict the prognosis of patients with hepatic alveolar echinococcosis. The higher the peripheral blood PNI before surgery, the better the prognosis of patients. -
Key words:
- Echinococcosis, Hepatic /
- Prognostic Nutritional Index /
- Prognosis
-
包虫病是由棘球绦虫的幼虫寄生于人体引起的一类以人为中间宿主的人兽共患寄生虫病,主要分为囊型包虫病和泡型包虫病两大类,约70%发生于肝脏,20%发生于肺部[1-3]。肝泡型包虫病的发病率较囊型包虫病稍低,但因其类似于恶性肿瘤样的生长特点,对人体的致病性强、致死率高。目前,肝包虫病的治疗方法以外科治疗为主,辅以化疗等综合治疗,但因包虫病的生长较为缓慢且早期症状不明显,大多数患者错过最佳治疗阶段,从而导致手术效果不佳且预后差。因此,探索有效预测肝泡型包虫病患者的预后指标并且制订个体化治疗方案至关重要。预后营养指数(prognostic nutritional index,PNI)由Buzby等[4]于1980年首次提出,常被用于评估患者术前营养状况和免疫功能[5-7]。近年来,其也被用作癌症患者的临床预后评估[8-9]。但关于PNI与肝泡型包虫病预后的关系研究较少,因此,本研究通过研究PNI与肝泡型包虫病的关系及患者预后相关影响因素,旨在为肝泡型包虫病的临床诊断和治疗提供参考。
1. 资料与方法
1.1 研究对象
回顾性分析2015年1月—2018年12月青海大学附属医院肝胆胰外科手术治疗的242例肝泡型包虫病患者的临床资料。手术方式包括肝包虫根治术、姑息性手术治疗。纳入标准:(1)术前经B超、CT或MRI等影像学检查诊断为肝泡型包虫病;(2)术前无针对性应用阿苯达唑类抗虫药治疗;(3)无严重合并症,能耐受手术治疗;(4)术前肝功能Child-Pugh分级为A或B级者。排除标准:(1)合并肝硬化、肝脏肿瘤患者;(2)拒绝手术治疗者;(3)病历资料缺失或失访患者。
1.2 分析方法
收集患者术前1周血常规及肝功能结果,计算PNI,PNI=Alb(g/L)+5×外周血淋巴细胞计数(×109 /L)。根据患者PNI最佳临界值分为高PNI组和低PNI组。
1.3 随访
通过查阅门诊病例、电话等方式进行随访,3~6个月随访1次。随访内容包括体格检查、肝泡型包虫有无复发及复发的时间、死亡患者死亡的时间及原因。总生存期定义为从手术日期到包虫病相关死亡或随访截止时间,随访截止时间为2020年5月或患者死亡。
1.4 伦理学审查
本研究方案经由青海大学附属医院伦理委员会审批,批号:PSL2018006,并取得所有受试者本人或家属的知情同意。
1.5 统计学方法
采用SPSS 22.0统计软件进行数据分析。计数资料两组间比较采用χ2检验。PNI的cut-off值通过X-Tile(Yale University, New Haven, CT, USA)确定[10]。Kaplan-Meier法绘制生存曲线,组间差异比较采用log-rank检验。采用Cox回归模型进行单因素和多因素分析,并计算风险比(HR)及对应的95%CI。P < 0.05为差异有统计学意义。
2. 结果
2.1 一般资料
242例患者中男96例(39.7%),女146例(60.3%),年龄11.0~67.0岁,平均(36.6±11.7)岁。242例患者术后均顺利出院,无死亡患者。所有患者中接受肝泡型包虫病根治术治疗187例(77.3%),接受姑息性治疗55例(22.7%)。患者中位随访时间45个月,随访结束时,死亡70例(28.9%),存活172例(71.1%)。
2.2 PNI与肝泡型包虫病患者临床病理因素的关系
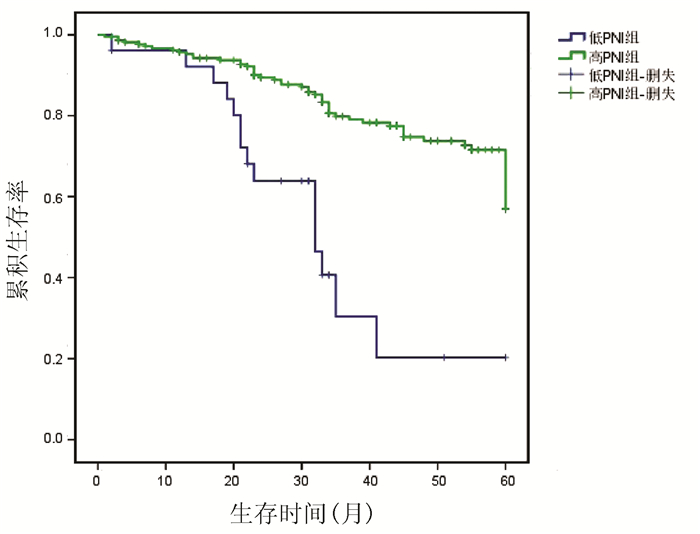
PNI的临界值为32.4(图 1),PNI≤32.4为低PNI组(n=26),PNI> 32.4为高PNI组(n=216)。PNI与治疗方式、术中出血量、包虫分期、病灶数、Child-Pugh分级、AST、TBil、Alb、ALP、PT、淋巴细胞计数均有关(P值均 < 0.05)(表 1)。
表 1 PNI与肝泡型包虫病患者临床病理因素的关系因素 高PNI组(n=216) 低PNI组(n=26) χ2值 P值 因素 高PNI组(n=216) 低PNI组(n=26) χ2值 P值 年龄[例(%)] 2.004 0.157 AST[例(%)] 3.948 0.047 ≤30岁 71(32.9) 5(19.2) ≤40 U/L 111(51.4) 8(30.8) >30岁 145(67.1) 21(80.8) >40 U/L 105(48.6) 18(69.2) 性别[例(%)] 1.366 0.242 TBil[例(%)] 9.249 0.002 男 82(38.1) 13(50.0) ≤34.2 μmol/L 126(58.3) 7(26.9) 女 133(61.9) 13(50.0) >34.2 μmol/L 90(41.7) 19(73.1) 治疗方式[例(%)] 42.049 < 0.001 根治性治疗 180(83.3) 7(26.9) Alb[例(%)] 15.487 < 0.001 姑息治疗 36(16.7) 19(73.1) ≤35 g/L 132(61.1) 26(100.0) 术中出血量[例(%)] 12.114 < 0.001 >35 g/L 84(38.9) 0 < 1000 ml 135(62.5) 7(26.7) ALP[例(%)] 8.367 0.004 ≥1000 ml 81(37.5) 19(73.3) ≤150 U/L 54(25.0) 0 包虫分期1)[例(%)] 18.529 < 0.001 >150 U/L 162(75.0) 26(100.0) 早、中期 113(52.3) 2(7.7) PT[例(%)] 8.324 0.004 晚期 103(47.7) 24(92.3) ≤16 s 106(49.1) 5(19.2) 有无转移[例(%)] 1.593 0.207 有 168(77.8) 23(88.5) >16 s 110(50.9) 21(80.8) 无 48(22.2) 3(11.5) 中性粒细胞[例(%)] 0.536 0.464 病灶数[例(%)] 5.729 0.017 ≤6.3×109/L 199(92.6) 23(88.5) 单发 135(62.8) 10(38.5) >6.3×109/L 16(7.4) 3(11.5) 多发 80(32.7) 16(61.5) 血小板[例(%)] 2.968 0.085 Child-Pugh分级[例(%)] 18.199 < 0.001 ≤300×109/L 111(51.4) 18(69.2) A级 103(47.7) 1(3.8) >300×109/L 105(48.6) 8(30.8) B级 113(52.3) 25(96.2) 并发症[例(%)] 2.534 0.111 淋巴细胞[例(%)] 8.509 0.004 有 114(52.8) 18(69.2) ≤0.8×109/L 10(4.6) 5(19.2) 无 102(47.2) 8(30.8) >0.8×109/L 206(95.4) 21(80.8) ALT[例(%)] 0.001 0.975 白细胞[例(%)] 1.364 0.243 ≤40 U/L 99(45.8) 12(46.2) ≤10×109/L 185(85.6) 20(76.9) >40 U/L 117(54.2) 14(53.8) >10×109/L 31(14.4) 6(23.1) 注:1)根据影像学检查,肝泡型包虫病在肝内侵袭范围以及临床表现,将肝泡型包虫病分为早、中、晚3期。早期病灶局限于1个肝段,中期病灶侵犯≥2个肝段,晚期合并多种并发症或脏器转移。 2.3 PNI与肝泡型包虫病患者总生存期的关系
高PNI组患者平均生存时间为51.355个月(95%CI:49.044~53.666),低PNI组患者平均生存时间为33.783个月(95%CI:26.450~51.355)。低PNI组1、3、5年生存率分别为92.1%、30.5%、20.3%,高PNI组分别为95.7%、79.5%、56.9%,高PNI组患者生存率优于低PNI组(χ2=24.626,P < 0.001)(图 2)。
2.4 Cox回归分析
单因素分析结果显示,治疗方式、术中出血量、包虫分期、PNI、Child-Pugh分级、并发症、ALP、PT、中性粒细胞及血小板计数均是影响肝泡型包虫病患者预后的因素(P值均 < 0.05)。将以上因素纳入多因素Cox回归模型,结果显示,治疗方式、术中出血量、PNI、并发症、中性粒细胞是影响肝泡型包虫病患者预后的独立危险因素(P值均 < 0.05)(表 2)。
表 2 影响肝泡型包虫患者生存的单因素及多因素分析因素 单因素分析 多因素分析 HR(95%CI) P值 HR(95%CI) P值 年龄(≤30岁=1; >30岁=2) 1.272(0.744~2.175) >0.05 性别(男=1;女=2) 1.268(0.791~2.033) >0.05 治疗方式(根治手术=1; 姑息治疗=2) 3.195(1.973~5.176) < 0.001 1.461(1.132~1.890) 0.004 术中出血量(< 1000 ml=1; ≥1000 ml=2) 6.832(3.893~11.990) < 0.001 6.078(3.412~10.826) < 0.001 包虫分期(早期=0; 中、晚期=2) 3.841(2.017~7.318) < 0.05 转移(有=0; 无=1) 1.430(0.731~2.797) >0.05 病灶数(< 单发=1;多发=2) 1.104(0.688~1.773) >0.05 PNI(≤32.4=1; >32.4=2) 0.254(0.141~0.457) < 0.001 0.367(0.192~0.701) 0.002 Child~Pugh分级(A级=1;B级=2) 3.066(2.716~6.449) < 0.05 并发症(无=0; 有=1) 2.278(1.398~3.711) < 0.05 2.007(1.204~3.346) 0.008 ALT(≤40 U/L=1; >40 U/L=2) 1.201(0.748~1.931) >0.05 AST(≤40 U/L=1; >40 U/L=2) 1.436(0.894~2.307) >0.05 TBil(≤32.4 μmol/L=1; >32.4 μmol/L=2) 1.572(0.983~2.514) >0.05 ALP(≤150 U/L=1; >150 U/L=2) 2.237(1.180~4.790) < 0.05 PT(≤16=1; >16=2) 1.824(1.113~2.991) < 0.05 中性粒细胞(≤7×109/L =1; >7×109/L=2) 2.533(1.210~5.304) < 0.05 2.772(1.304~5.891) 0.008 血小板(≤300×109/L=1; >300×109/L=2) 2.016(1.248~3.257) < 0.05 1.609(0.969~2.672) 0.066 白细胞(≤10×109/L=1; >10×109/L=2) 1.486(0.797~2.770) >0.05 3. 讨论
近年来,相关研究表明,营养状态和免疫功能在肿瘤的发生发展过程中扮演着重要的角色,患者营养状况、免疫功能与治疗的选择以及生存质量存在一定的关联,因此监测机体营养、免疫状态对疗效和预后具有一定的指导意义[11]。PNI主要通过血清Alb以及外周血淋巴细胞计数计算获得,可综合反映患者的营养状态和免疫状态[12-13]。Flavill等[14]首次提出其在胃肠道肿瘤患者的术前营养、免疫功能以及手术风险评估中的作用。最近的研究[15-18]表明,PNI与结直肠癌、肝细胞癌、食管癌等预后密切相关。
传统制定临界值的途径包括查找相关参考文献、指南共识或ROC曲线等,而X-Tile虽然类似于ROC曲线,但其考虑了时间因素(一般关于预后的研究都需考虑患者的生存时间),且可以直观地计算出最佳截点,从而更可能得出阳性结果(统计学差异)。本研究中,通过X-Tile软件寻找PNI的临界值为32.4,并以此数值为界分析PNI与肝泡型包虫病患者预后的关系,发现PNI可作为预后评估指标。虽然这与之前研究报道的cut-off值不一样,但是此cut-off值比较可靠,且考虑了患者的生存时间。
通过分析PNI与肝泡型包虫病患者总生存期的关系,结果提示术前低水平PNI的肝泡型包虫病患者生存期明显缩短,5年生存率显著低于高PNI组(P < 0.001)。PNI影响肝泡型包虫病患者预后可能包括以下几个方面:(1)肝脏是合成Alb的主要场所,同时其作为血浆蛋白的主要成分,是人体内一种重要物质,可以反映患者营养状况,是患者预后的独立影响因素,同时也是一种解毒剂和转运蛋白,已被广泛应用于评估疾病进展和预测患者生存情况[19]。肝泡型包虫患者血清中高表达的IL-1、IL-6以及TNFα可抑制Alb的合成,这也导致了低Alb血症[20]。(2) 淋巴细胞在机体免疫监测和免疫介导中发挥重要的作用,患者刚开始感染包虫时,淋巴细胞可以诱导相关细胞因子的分泌以及参与机体免疫系统杀伤棘球蚴绦虫,并抑制虫体的生长和远处转移,随后肝包虫可通过分泌抗原及利用表面分子,诱导肝脏特异性CD4+和CD8+效应T淋巴细胞表面的PD-1表达升高,并通过PD-1/PD-L1通路使T淋巴细胞数目减少,抑制增殖能力及细胞因子的分泌(如IL-2、IFNγ、IL-10),从而导致肝泡蚴的免疫逃逸,使其得以在机体内长期生存[21],淋巴细胞减少代表着宿主的免疫抑制状态,可导致不良预后。因此,结合二者的PNI可同时反映肝泡型包虫病患者免疫和营养状况,这些均可能是低水平PNI肝泡型包虫病患者预后不良的主要原因。本研究结果显示,治疗方式、术中出血量、PNI、并发症、中性粒细胞是影响肝泡型包虫病患者预后的独立危险因素,而包虫分期、Child-Pugh分级、ALP、PT不是肝泡型包虫病的独立预后因素,但仍然能够作为预测患者生存时间的重要参考指标。
综上所述,PNI作为一种新的、准确的、客观的生物预测标志物,因其具备简单、便于计算、通用性、无创性、廉价等优势,有望成为评估肝泡型包虫病患者预后的评估指标。
-
表 1 PNI与肝泡型包虫病患者临床病理因素的关系
因素 高PNI组(n=216) 低PNI组(n=26) χ2值 P值 因素 高PNI组(n=216) 低PNI组(n=26) χ2值 P值 年龄[例(%)] 2.004 0.157 AST[例(%)] 3.948 0.047 ≤30岁 71(32.9) 5(19.2) ≤40 U/L 111(51.4) 8(30.8) >30岁 145(67.1) 21(80.8) >40 U/L 105(48.6) 18(69.2) 性别[例(%)] 1.366 0.242 TBil[例(%)] 9.249 0.002 男 82(38.1) 13(50.0) ≤34.2 μmol/L 126(58.3) 7(26.9) 女 133(61.9) 13(50.0) >34.2 μmol/L 90(41.7) 19(73.1) 治疗方式[例(%)] 42.049 < 0.001 根治性治疗 180(83.3) 7(26.9) Alb[例(%)] 15.487 < 0.001 姑息治疗 36(16.7) 19(73.1) ≤35 g/L 132(61.1) 26(100.0) 术中出血量[例(%)] 12.114 < 0.001 >35 g/L 84(38.9) 0 < 1000 ml 135(62.5) 7(26.7) ALP[例(%)] 8.367 0.004 ≥1000 ml 81(37.5) 19(73.3) ≤150 U/L 54(25.0) 0 包虫分期1)[例(%)] 18.529 < 0.001 >150 U/L 162(75.0) 26(100.0) 早、中期 113(52.3) 2(7.7) PT[例(%)] 8.324 0.004 晚期 103(47.7) 24(92.3) ≤16 s 106(49.1) 5(19.2) 有无转移[例(%)] 1.593 0.207 有 168(77.8) 23(88.5) >16 s 110(50.9) 21(80.8) 无 48(22.2) 3(11.5) 中性粒细胞[例(%)] 0.536 0.464 病灶数[例(%)] 5.729 0.017 ≤6.3×109/L 199(92.6) 23(88.5) 单发 135(62.8) 10(38.5) >6.3×109/L 16(7.4) 3(11.5) 多发 80(32.7) 16(61.5) 血小板[例(%)] 2.968 0.085 Child-Pugh分级[例(%)] 18.199 < 0.001 ≤300×109/L 111(51.4) 18(69.2) A级 103(47.7) 1(3.8) >300×109/L 105(48.6) 8(30.8) B级 113(52.3) 25(96.2) 并发症[例(%)] 2.534 0.111 淋巴细胞[例(%)] 8.509 0.004 有 114(52.8) 18(69.2) ≤0.8×109/L 10(4.6) 5(19.2) 无 102(47.2) 8(30.8) >0.8×109/L 206(95.4) 21(80.8) ALT[例(%)] 0.001 0.975 白细胞[例(%)] 1.364 0.243 ≤40 U/L 99(45.8) 12(46.2) ≤10×109/L 185(85.6) 20(76.9) >40 U/L 117(54.2) 14(53.8) >10×109/L 31(14.4) 6(23.1) 注:1)根据影像学检查,肝泡型包虫病在肝内侵袭范围以及临床表现,将肝泡型包虫病分为早、中、晚3期。早期病灶局限于1个肝段,中期病灶侵犯≥2个肝段,晚期合并多种并发症或脏器转移。 表 2 影响肝泡型包虫患者生存的单因素及多因素分析
因素 单因素分析 多因素分析 HR(95%CI) P值 HR(95%CI) P值 年龄(≤30岁=1; >30岁=2) 1.272(0.744~2.175) >0.05 性别(男=1;女=2) 1.268(0.791~2.033) >0.05 治疗方式(根治手术=1; 姑息治疗=2) 3.195(1.973~5.176) < 0.001 1.461(1.132~1.890) 0.004 术中出血量(< 1000 ml=1; ≥1000 ml=2) 6.832(3.893~11.990) < 0.001 6.078(3.412~10.826) < 0.001 包虫分期(早期=0; 中、晚期=2) 3.841(2.017~7.318) < 0.05 转移(有=0; 无=1) 1.430(0.731~2.797) >0.05 病灶数(< 单发=1;多发=2) 1.104(0.688~1.773) >0.05 PNI(≤32.4=1; >32.4=2) 0.254(0.141~0.457) < 0.001 0.367(0.192~0.701) 0.002 Child~Pugh分级(A级=1;B级=2) 3.066(2.716~6.449) < 0.05 并发症(无=0; 有=1) 2.278(1.398~3.711) < 0.05 2.007(1.204~3.346) 0.008 ALT(≤40 U/L=1; >40 U/L=2) 1.201(0.748~1.931) >0.05 AST(≤40 U/L=1; >40 U/L=2) 1.436(0.894~2.307) >0.05 TBil(≤32.4 μmol/L=1; >32.4 μmol/L=2) 1.572(0.983~2.514) >0.05 ALP(≤150 U/L=1; >150 U/L=2) 2.237(1.180~4.790) < 0.05 PT(≤16=1; >16=2) 1.824(1.113~2.991) < 0.05 中性粒细胞(≤7×109/L =1; >7×109/L=2) 2.533(1.210~5.304) < 0.05 2.772(1.304~5.891) 0.008 血小板(≤300×109/L=1; >300×109/L=2) 2.016(1.248~3.257) < 0.05 1.609(0.969~2.672) 0.066 白细胞(≤10×109/L=1; >10×109/L=2) 1.486(0.797~2.770) >0.05 -
[1] WANG WT, YANG C, YAN LN. New concept and strategy of surgical radical treatment of hepatic alveolar echinococcosis[J]. Natl Med J China, 2018, 98(38): 3049-3051. DOI: 10.3760/cma.j.issn.0376-2491.2018.38.001.王文涛, 杨闯, 严律南. 肝泡型包虫病外科根治性治疗的新理念与策略[J]. 中华医学杂志, 2018, 98(38): 3049-3051. DOI: 10.3760/cma.j.issn.0376-2491.2018.38.001. [2] NABULAJIANG A, TUERGANAILI A. Current status of research on laparoscopic treatment of hepatic cystic echinococcosis[J]. J Clin Hepatol, 2020, 36(11): 2613-2616. DOI: 10.3969/j.issn.1001-5256.2020.11.049.那布拉江·艾合买提, 吐尔干艾力·阿吉. 腹腔镜治疗肝囊型包虫病的研究现状[J]. 临床肝胆病杂志, 2020, 36(11): 2613-2616. DOI: 10.3969/j.issn.1001-5256.2020.11.049. [3] Chinese Doctor Association, Chinese College of Surgeons(CCS), Chinese Committee for Hadytidology(CCH). Expert consensus on diagnosis and treatment of hepatic cystic and alveolar echinococcosis (2019 edition)[J]. Chin J Dig Surg, 2019, 18(8): 711-721. DOI: 10.3760/cma.j.issn.1673-9752.2019.08.002.中国医师协会外科医师分会包虫病外科专业委员会. 肝两型包虫病诊断与治疗专家共识(2019版)[J]. 中华消化外科杂志, 2019, 18(8): 711-721. DOI: 10.3760/cma.j.issn.1673-9752.2019.08.002. [4] BUZBY GP, MULLEN JL, MATTHEWS DC, et al. Prognostic nutritional index in gastrointestinal surgery[J]. Am J Surg, 1980, 139(1): 160-167. DOI: 10.1016/0002-9610(80)90246-9. [5] SCHWEGLER I, von HOLZEN A, GUTZWILLER JP, et al. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer[J]. Br J Surg, 2010, 97(1): 92-97. DOI: 10.1002/bjs.6805. [6] KWAG SJ, KIM JG, KANG WK, et al. The nutritional risk is a independent factor for postoperative morbidity in surgery for colorectal cancer[J]. Ann Surg Treat Res, 2014, 86(4): 206-211. DOI: 10.4174/astr.2014.86.4.206. [7] STOTZ M, PICHLER M, ABSENGER G, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage Ⅲ colon cancer[J]. Br J Cancer, 2014, 110(2): 435-440. DOI: 10.1038/bjc.2013.785. [8] ONODERA T, GOSEKI N, KOSAKI G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients[J]. Nihon Geka Gakkai Zasshi, 1984, 85(9): 1001-1005. [9] HU WH, EISENSTEIN S, PARRY L, et al. Preoperative malnutrition with mild hypoalbuminemia associated with postoperative mortality and morbidity of colorectal cancer: A propensity score matching study[J]. Nutr J, 2019, 18(1): 33. DOI: 10.1186/s12937-019-0458-y. [10] CAMP RL, DOLLED-FILHART M, RIMM DL. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization[J]. Clin Cancer Res, 2004, 10(21): 7252-7259. DOI: 10.1158/1078-0432.CCR-04-0713. [11] PASTORE CA, ORLANDI SP, GONZALEZ MC. The Inflammatory-Nutritional Index; assessing nutritional status and prognosis in gastrointestinal and lung cancer patients[J]. Nutr Hosp, 2014, 29--(3): 629-634. DOI: 10.3305/nh.2014.29.3.7195. [12] SONG SB, LIU HG, XUE YW. Clinical significance of prognostic nutritional index in patients with advanced gastric cancer[J]. Chin J Gastrointestinal Surg, 2018, 21(2): 180-184. DOI: 10.3760/cma.j.issn.1671-0274.2018.02.012.宋书彬, 刘宏刚, 薛英威. 进展期胃癌患者预后营养指数的临床意义[J]. 中华胃肠外科杂志, 2018, 21(2): 180-184. DOI: 10.3760/cma.j.issn.1671-0274.2018.02.012. [13] ZHANG GL, LUO JG, SUN YS, et al. The relationship between the expression of ubiquitin in muscle tissue of patients with gastric cancer and nutritional status and prognosis[J]. Chin J Clin Nutrition, 2018, 26(2): 78-82. DOI: 10.3760/cma.j.issn.1674-635X.2018.02.003.章国良, 罗钧刚, 孙元水, 等. 胃癌患者肌肉组织中泛素表达与营养状况和预后的关系[J]. 中华临床营养杂志, 2018, 26(2): 78-82. DOI: 10.3760/cma.j.issn.1674-635X.2018.02.003. [14] FLAVILL E, FANG YV, MILES B, et al. Induction chemotherapy followed by concurrent chemoradiotherapy for advanced stage oropharyngeal squamous cell carcinoma with HPV and P16 testing[J]. Ann Otol Rhinol Laryngol, 2014, 123(5): 365-373. DOI: 10.1177/0003489414526685. [15] PINATO DJ, NORTH BV, SHARMA R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: The prognostic nutritional index (PNI)[J]. Br J Cancer, 2012, 106(8): 1439-1445. DOI: 10.1038/bjc.2012.92. [16] MOHRI Y, INOUE Y, TANAKA K, et al. Prognostic nutritional index predicts postoperative outcome in colorectal cancer[J]. World J Surg, 2013, 37(11): 2688-2692. DOI: 10.1007/s00268-013-2156-9. [17] WU JW, YU XJ, YANG QA. Association between Onodera's prognostic nutrition index and adverse effects of concurrent chemoradiotherapy for esophageal squamous cell carcinoma[J]. China Oncology, 2020, 30(7): 525-530. DOI: 10.19401/j.cnki.1007-3639.2020.07.007.吴婧文, 于学娟, 杨秋安. 小野寺预后营养指数与食管鳞癌同步放化疗不良反应的相关性研究[J]. 中国癌症杂志, 2020, 30(7): 525-530. DOI: 10.19401/j.cnki.1007-3639.2020.07.007. [18] YANG XJ, SHEN H, YUAN W, et al. Effect of supplemental parenteral nutrition on the pre-operative system immune inflammation index and postoperative prognostic nutrition index in esophageal cancer[J]. J Clin Exp Med, 2020, 19(20): 2202-2205. DOI: 10.3969/j.issn.1671-4695.2020.20.022.杨小娟, 沈慧, 袁伟, 等. 补充性肠外营养对食管癌术前系统免疫炎症指数及术后预后营养指数的影响[J]. 临床和实验医学杂志, 2020, 19(20): 2202-2205. DOI: 10.3969/j.issn.1671-4695.2020.20.022. [19] MANTZOROU M, KOUTELIDAKIS A, THEOCHARIS S, et al. Clinical value of nutritional status in cancer: What is its impact and how it affects disease progression and prognosis?[J]. Nutr Cancer, 2017, 69(8): 1151-1176. DOI: 10.1080/01635581.2017.1367947. [20] SHAO J, WANG ZX, WANG H, et al. Antibody microarray analysis of the serum inflammatory cytokines in patients with hepatic alveolar echinococcosis[J]. Chin J Gastroenterol Hepatol, 2017, 26(5): 566-569. DOI: 10.3969/j.issn.1006-5709.2017.05.024.邵军, 王志鑫, 王虎, 等. 泡型肝包虫病患者血清炎症因子的抗体芯片检测及分析[J]. 胃肠病学和肝病学杂志, 2017, 26(5): 566-569. DOI: 10.3969/j.issn.1006-5709.2017.05.024. [21] ZHANG F, PANG N, ZHU Y, et al. CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells are positively correlated with levels of IL-21 in active and transitional cystic echinococcosis patients[J]. BMC Infect Dis, 2015, 15: 457. DOI: 10.1186/s12879-015-1156-9. 期刊类型引用(5)
1. 何宏材,苏鹏宇,侯立朝,樊海宁. 红细胞分布宽度与血小板计数比值和血小板-白蛋白-胆红素评分预测肝脏多房棘球蚴病术后并发症的列线图构建. 中国普外基础与临床杂志. 2023(07): 795-801 .  百度学术
百度学术2. 杨金艳,李飞飞,谢元元,穆亚娟,韩瑞瑞,王蒙,高晓霞. 肝硬化上消化道出血患者预后营养指数与病情严重程度和短期预后相关性研究. 陕西医学杂志. 2023(11): 1530-1534 .  百度学术
百度学术3. 朱青,马洁,戴尧,庞明泉,樊海宁,崔红元. 多房棘球蚴病患者营养评估及干预措施. 中华肝脏外科手术学电子杂志. 2023(03): 356-359 .  百度学术
百度学术4. 何桥,原文聪,樊海宁,任宾. 红细胞分布宽度对肝泡型包虫病术后预测价值分析. 实用医院临床杂志. 2022(01): 46-50 .  百度学术
百度学术5. 薛红,刘先进,明芳,章颖,邵建国,卞兆连. 预后营养指数与乙型肝炎病毒相关慢加急性肝衰竭患者预后的关系. 实用医学杂志. 2022(10): 1240-1245 .  百度学术
百度学术其他类型引用(3)
-




 PDF下载 ( 2428 KB)
PDF下载 ( 2428 KB)


 下载:
下载:


 下载:
下载:
 百度学术
百度学术