盐酸可洛派韦胶囊在HCV感染者中的耐受性和药代动力学分析
DOI: 10.3969/j.issn.1001-5256.2021.06.018
Tolerance and pharmacokinetics of coblopasvir hydrochloride capsules in patients with hepatitis C virus infection
-
摘要:
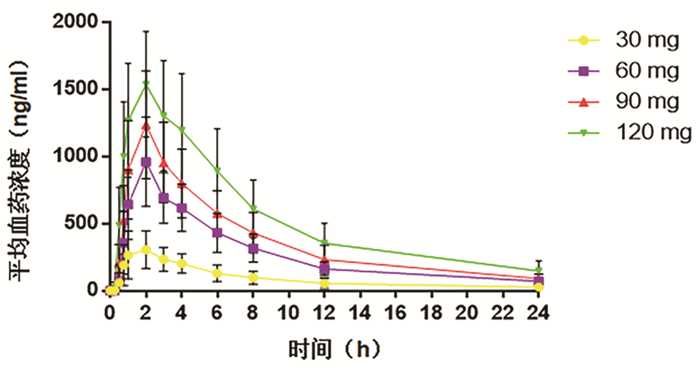
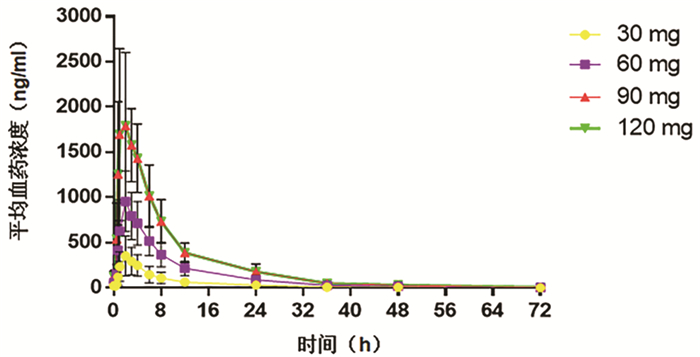
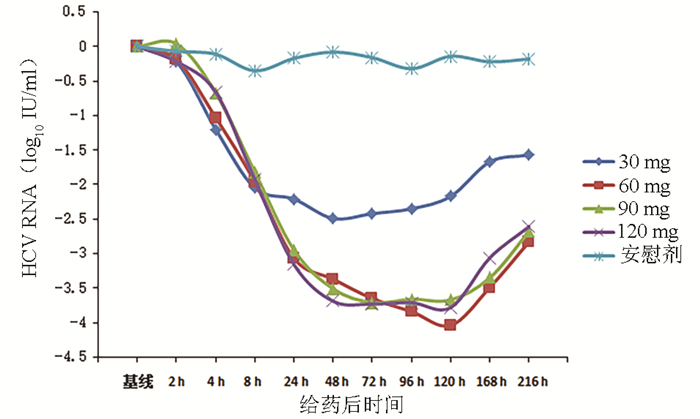
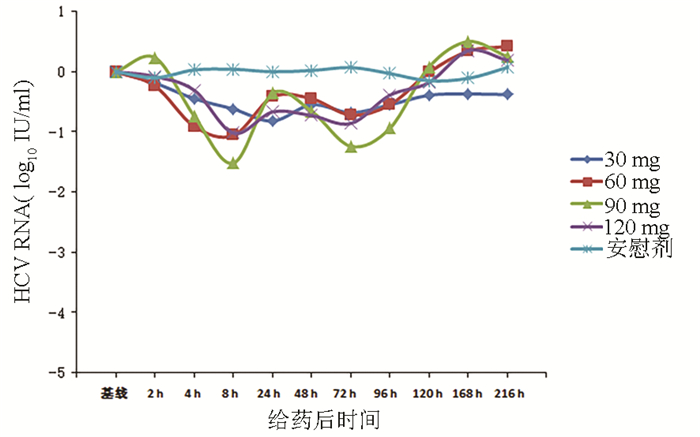
目的 评价盐酸可洛派韦胶囊在丙型肝炎患者中的耐受性、药代动力学和抗病毒活性学特征。 方法 选取2016年11月—2017年1月吉林大学第一医院收治的36例丙型肝炎患者作为研究对象,预设4个剂量(30、60、90、120 mg)及安慰剂组。受试者连续给药3 d,1次/d;在D2和D6进行耐受性评价;D8、D10进行随访。受试者采用单剂量爬坡的方式依次入组,在单次给药耐受的前提下进行多次给药研究。采用串联质谱法(LC-MS/MS)法测定盐酸可洛派韦在人体中的血药浓度,用WinNonlin 6.4软件计算主要药代动力学参数。用HCV RNA载量评估不同时间点的抗病毒活性,多组比较采用单因素方差分析,进一步两组间比较采用LSD-t检验。 结果 每日单次口服30~120 mg盐酸可洛派韦胶囊,盐酸可洛派韦在人体内血药浓度和药物暴露量随着剂量的增加而增加。空腹多次给药与单次给药相比,血药浓度和药物暴露量没有显著差异,且在人体内没有蓄积。每日1次口服盐酸可洛派韦胶囊后,HCV 1b型受试者HCV RNA载量自基线起开始下降,120 h下降达最大水平,各剂量组间抗病毒活性差异有统计学意义(F=14.621,P<0.000 1),其中60 mg组下降较30 mg更明显(P=0.025),但与90 mg和120 mg组相比无统计学差异(P值均>0.05)。各组HCV 2a型受试者服药后HCV RNA载量无统计学差异(P值均>0.05)。共有20例受试者报告了34例次治疗期不良事件,其中与研究用药有关的治疗期不良事件19例次,未发生重要不良事件及严重不良事件。 结论 空腹口服盐酸可洛派韦胶囊30~120 mg/d (持续3 d)具有良好的耐受性和抗病毒活性,在治疗HCV感染者方面具有广阔的应用前景,也为其Ⅱ期研究的剂量选择提供依据。 Abstract:Objective To investigate the tolerance, pharmacokinetics, and antiviral activity of coblopasvir hydrochloride capsules in patients with hepatitis C. Methods A total of 36 patients with hepatitis C who were admitted to The First Hospital of Jilin University from November 2016 to January 2017 were enrolled as subjects, and four dose groups (30 mg, 60 mg, 90 mg, and 120 mg) and one placebo group were established. The subjects were administered once daily for 3 consecutive days; tolerance was evaluated on D2 and D6, and follow-up was performed on D8 and D10. The subjects were enrolled based on single dose escalation, and a multiple-dose study was conducted under the premise of good tolerance to single dose. Liquid chromatography-tandem mass spectrometry was used to measure the plasma concentration of coblopasvir hydrochloride in human body, and WinNonlin 6.4 software was used to calculate main pharmacokinetic parameters. HCV RNA load was used to evaluate antiviral activity at different time points; a one-way analysis of variance was used for comparison between multiple groups, and the LSD t-test was used for further comparison between two groups. Results After coblopasvir hydrochloride capsules were administered orally once a day at a dose of 30-120 mg, the plasma concentration and exposure of coblopasvir hydrochloride increased with the increase in dose. There were no significant differences in plasma concentration and exposure between multiple-dose administration and single-dose administration in a fasting state, without accumulation in human body. After the oral administration of coblopasvir hydrochloride capsules once a day, the subjects with HCV genotype 1b had a reduction in HCV RNA load since baseline, with the lowest level at 120 hours, and there was a significant difference in antiviral activity between different dose groups (F=14.621, P < 0.000 1), among which the 60 mg group had a significantly greater reduction than the 30 mg group (P=0.025), while there was no significant difference between the 60 mg group and the 90/120 mg group (P > 0.05). There was no significant difference in HCV RNA load between different groups of patients with HCV genotype 2a (P > 0.05). Of all 36 subjects, 20 reported 34 cases of treatment-emergent adverse events, among which 19 cases were associated with coblopasvir hydrochloride, and no significant adverse events or serious adverse events were observed. Conclusion Oral administration of coblopasvir hydrochloride capsules in a fasting state at a dose of 30-120 mg/d (for 3 consecutive days) has good safety and antiviral activity. Therefore, it has good application prospect in the treatment of HCV infection and provides a basis for dose selection in phrase 2 study. -
Key words:
- Hepatitis C /
- Antiviral Agents /
- Pharmacokinetics
-
表 1 受试者人口学资料和基线特征
项目 30 mg组
(n=8)60 mg组
(n=8)90 mg组
(n=6)120 mg组
(n=6)安慰剂组
(n=8)男/女(例) 6/2 5/3 3/3 4/2 5/3 年龄(岁) 53.61±5.58 50.13±8.97 47.70±8.21 55.80±6.52 49.32±6.98 BMI(kg/m2) 24.62±2.08 23.90±1.73 23.93±2.81 23.25±2.56 23.91±3.04 HCV RNA (log10 IU/ml) 6.26±0.30 6.17±0.43 6.09±0.64 6.24±0.51 6.38±0.43 HCV基因型(1b/2a, 例) 3/5 5/3 5/1 3/3 6/2 表 2 受试药物单次给药药代动力学参数
药代参数 30 mg组
(n=8)60 mg组
(n=8)90 mg组
(n=6)120 mg组
(n=6)Tmax (h) 1.75±0.71 2.00±0.00 1.83±0.41 1.50±0.55 Cmax (ng/ml) 327.35±146.31 961.72±332.56 1241.00±398.61 1580.00±381.60 AUC0-24(h*ng/ml) 2234±1140 6634±2050 8984±2999 13 135±4218 AUC0-∞(h*ng/ml) 2598±1455 7325±2204 9871±3406 14 845±5275 λz (1/h) 0.09±0.03 0.10±0.01 0.11±0.01 0.09±0.02 t1/2 (h) 7.78±2.02 6.87±0.91 6.63±0.62 7.63±1.50 Vd/F (L) 153.20±53.72 90.22±38.87 93.52±23.29 96.05±32.90 CL/F (L/h) 15.48±8.76 8.91±2.91 9.97±3.04 8.99±3.16 MRT (h) 10.32±2.41 9.61±0.87 9.33±0.69 10.15±1.80 表 3 受试药物多次给药药代动力学参数
药代参数 30 mg组
(n=8)60 mg组
(n=8)90 mg组
(n=6)120 mg组
(n=6)Tmax (h) 2.25±0.46 2.13±0.35 1.83±0.41 1.83±0.75 Cmin, ss(ng/ml) 20.69±15.10 52.45±17.86 84.73±29.30 122.40±55.55 Cmax, ss(ng/ml) 360.91±211.42 975.42±335.41 1420.00±449.72 1951.00±707.04 AUC0-24, ss(h*ng/ml) 2533±1497 7823±2698 11 201±3522 15 482±5189 AUC0-72, ss(h*ng/ml) 2913±1792 9013±3302 12 552±4138 17 851±6467 AUC0-∞, ss(h*ng/ml) 2932±1809 9073±3351 12 609±4165 18 063±6722 λz (1/h) 0.08±0.02 0.07±0.02 0.07±0.02 0.06±0.01 t1/2(h) 8.83±2.47 10.17±2.27 10.85±2.84 13.08±3.69 Vd/F (L) 211.00±234.60 105.73±33.43 118.92±40.84 131.21±35.01 CL/F (L/h) 15.50±12.00 7.53±2.94 7.98±3.17 7.32±2.26 MRT (h) 10.79±2.41 11.01±1.90 9.88±1.25 10.98±1.39 DF (%) 321.01±32.16 287.04±42.17 286.83±16.86 282.02±28.87 蓄积指数1 1.10±0.54 1.03±0.18 1.28±0.72 1.22±0.23 蓄积指数2 1.13±0.44 1.18±0.21 1.33±0.57 1.19±0.13 表 4 各类不良事件发生情况及比较
不良事件 30 mg 60 mg 90 mg 120 mg 安慰剂 例次 例数 发生率(%) 例次 例数 发生率(%) 例次 例数 发生率(%) 例次 例数 发生率(%) 例次 例数 发生率(%) 治疗期不良事件 12 6 75.00 15 7 87.50 1 1 16.67 3 3 50.00 3 3 37.50 与研究用药有关的治疗期不良事件 5 4 50.00 8 5 62.50 1 1 16.67 2 2 33.33 3 3 37.50 -
[1] Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of hepatitis C (2019 version)[J]. Chin J Hepatol, 2019, 27(12): 962-979. DOI: 10.3969/j.issn.1001-5256.2019.12.008.中华医学会肝病学分会, 中华医学会感染病学分会. 丙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2670-2686. DOI: 10.3969/j.issn.1001-5256.2019.12.008. [2] HORSLEY-SILVA JL, VARGAS HE. New therapies for hepatitis C virus infection[J]. Gastroenterol Hepatol (N Y), 2017, 13(1): 22-31. [3] WU R, GENG D, CHI X, et al. Computational analysis of naturally occurring resistance-associated substitutions in genes NS3, NS5A, and NS5B among 86 subtypes of hepatitis C virus worldwide[J]. Infect Drug Resist, 2019, 12: 2987-3015. DOI: 10.2147/IDR.S218584. [4] WEI L, OMATA M, LIM YS, et al. HCV phylogenetic signature and prevalence of pretreatment NS5A and NS5B NI-resistance associated substitutions in HCV-infected patients in Mainland China[J]. Antiviral Res, 2018, 158: 178-184. DOI: 10.1016/j.antiviral.2018.08.001. [5] ASSELAH T, MARCELLIN P, SCHINAZI RF. Treatment of hepatitis C virus infection with direct-acting antiviral agents: 100% cure?[J]. Liver Int, 2018, 38(Suppl 1): 7-13. DOI: 10.1111/liv.13673. 期刊类型引用(3)
1. 张艳培,梁健,邓鑫,梁明坤,张冰冰,徐粤娟. 浅析肝纤维化发生机制. 大众科技. 2021(02): 41-44+12 .  百度学术
百度学术2. 曾静,熊庆,张丽丽,袁娟,何莹,邱黎菲. SPACE肽修饰成纤维细胞生长因子21的制备及对小鼠皮肤瘢痕形成的抑制作用. 中国免疫学杂志. 2021(11): 1308-1312 .  百度学术
百度学术3. 唐青蓝,李红梅,张礼林,王玉丰,颜礼完,周君. 小鼠FGF21基因克隆及his-mFGF21融合蛋白的诱导表达. 贵州医科大学学报. 2019(05): 552-556 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 2093 KB)
PDF下载 ( 2093 KB)

 下载:
下载:
 百度学术
百度学术

