肝脾硬度联合血清腺苷脱氨酶对乙型肝炎肝硬化重度食管静脉曲张的预测价值分析
DOI: 10.3969/j.issn.1001-5256.2021.06.020
Value of liver/spleen stiffness combined with serum adenosine deaminase in predicting severe esophageal varices in patients with hepatitis B cirrhosis
-
摘要:
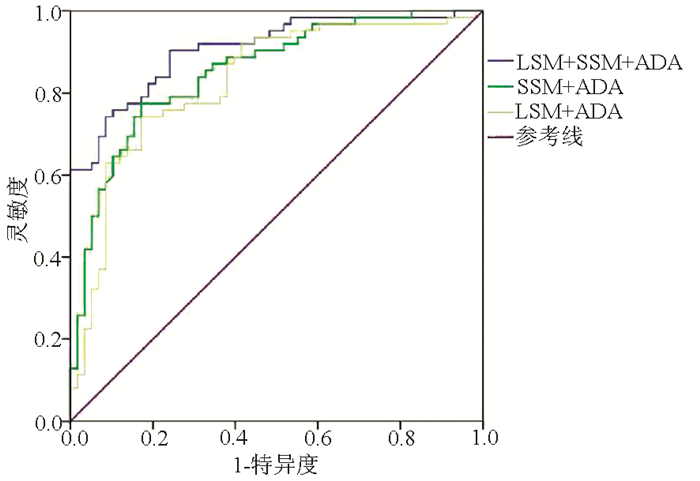
目的 评价FibroTouch(FT)瞬时弹性成像技术测量肝脾硬度及其联合血清腺苷脱氨酶(ADA)对乙型肝炎肝硬化患者重度食管静脉曲张(EV)的预判价值。 方法 选取2017年12月—2020年6月在长沙市第一医院感染科就诊的120例乙型肝炎肝硬化患者的临床资料,FT测量肝脏硬度值(LSM)和脾脏硬度值(SSM),完善电子胃镜、血清ADA、Hb、PLT、Alb、ALT及AST检查,计算血清肝纤维化指标APRI、AAR及FIB-4。将研究对象根据胃镜下EV程度,分为重度EV组(n=58)和非重度(无或轻中度)EV组(n=62)。满足正态分布的计量资料2组间比较采用t检验,非正态性的计量资料2组间比较采用Mann-Whitney U检验,计数资料2组间比较采用χ2检验。采用Spearman秩相关检验分析LSM、SSM、ADA与重度EV的关系。采用受试者工作特征曲线(ROC曲线)分析LSM、SSM、ADA诊断重度EV的效能,计算敏感度及特异度,行二分类变量logistic回归,计算联合指标的ROC曲线下面积(AUC),AUC比较应用Z检验。 结果 LSM、SSM与ADA在2组间比较差异均有统计学意义(P值均<0.05)。LSM、SSM、ADA与重度EV之间均存在显著正相关,相关系数分别为0.686、0.743和0.723(P值均<0.05)。LSM、SSM、ADA预判重度EV的最佳阈值分别为22.35 kPa、45.25 kPa和34.50 U/L,AUC分别为0.746、0.802和0.791,灵敏度分别为82.8%、75.9%和58.6%,特异度分别为65.6%、77.4%和90.2%。LSM+ADA、SSM+ADA、LSM+SSM+ADA联合预判重度EV的AUC分别为0.826、0.853和0.907(P值均<0.05)。 结论 肝脾硬度联合血清ADA对重度EV具有较好的预判价值,能够为临床上拒绝行胃镜检查患者预测重度EV提供初步诊断依据。 Abstract:Objective To investigate the value of liver stiffness measurement (LSM) and spleen stiffness measurement (SSM) based on FibroTouch (FT) transient elastography combined with serum adenosine deaminase (ADA) in predicting severe esophageal varices (EV) in patients with hepatitis B cirrhosis. Methods Related clinical data were collected from 120 patients with hepatitis B cirrhosis who attended Department of Infectious Diseases, Changsha First Hospital, from December 2017 to June 2020. FT was used to measure LSM and SSM, and related examinations were performed, including electronic gastroscopy and serum levels of ADA, hemoglobin, albumin, alanine aminotransferase, and aspartate aminotransferase and platelet count. The serum liver fibrosis markers aspartate aminotransferase-to-platelet ratio index (APRI), aspartate aminotransferase/alanine aminotransferase ratio (AAR), and fibrosis-4 (FIB-4) were calculated. According to the severity of EV under gastroscopy, the subjects were divided into severe EV group with 58 patients and non-severe EV (without EV or with mild-to-moderate EV) group with 62 patients. The t-test was used for comparison of normally distributed continuous data between groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between groups; the chi-square test was used for comparison of categorical data between groups. The Spearman rank correlation test was used to investigate the correlation of LSM, SSM, and ADA with severe EV. The receiver operating characteristic (ROC) curve was used to analyze the efficacy of LSM, SSM, and ADA in the diagnosis of severe EV, and sensitivity and specificity were calculated. A multivariate binary logistic regression analysis was performed to calculate the area under the ROC curve (AUC) of the combined indicators, and the Z test was used for comparison of AUC. Results There were significant differences in LSM, SSM, and ADA between the two groups (all P < 0.05). LSM, SSM, and ADA were positively correlated with severe EV, with a correlation coefficient of 0.686, 0.743, and 0.723, respectively (all P < 0.05). The optimal cut-off value was 22.35 kPa for LSM, 45.25 kPa for SSM, and 34.50 U/L for ADA in predicting severe EV, with an AUC of 0.746, 0.802, and 0.791, respectively, a sensitivity of 82.8%, 75.9%, and 58.6%, respectively, and a specificity of 65.6%, 77.4%, and 90.2%, respectively. LSM+ADA, SSM+ADA, and LSM+SSM+ADA had an AUC of 0.826, 0.853, and 0.907, respectively, in predicting severe EV (all P < 0.05). Conclusion Liver/spleen stiffness combined with serum ADA has a good value in predicting severe EV, which can provide a preliminary diagnostic basis for severe EV in patients who refuse to undergo gastroscopy. -
Key words:
- Hepatitis B /
- Liver Cirrhosis /
- Esophageal and Gastric Varices
-
表 1 两组患者各指标比较
指标 重度EV组(n=58) 非重度EV组(n=62) 统计值 P值 男/女(例) 48/10 54/8 χ2=0.44 0.506 年龄(岁) 52.41(47.05~57.77) 52.32(45.58~59.06) Z=0.08 0.935 病程(年) 5.48(3.12~7.84) 5.24(2.64~7.84) Z=0.52 0.602 LSM(kPa) 25.33(20.48~30.18) 22.28(19.10~25.46) Z=4.11 <0.001 SSM(kPa) 49.01(42.93~55.09) 43.38(39.81~46.94) Z=6.27 <0.001 PLT(×109/L) 67.01(49.45~84.62) 70.55(56.65~84.45) Z=1.22 0.224 Hb(g/L) 80.45(65.93~94.97) 83.42(73.7~93.14) Z=1.33 0.191 Alb(g/L) 28.82(25.34~32.30) 28.68(25.72~31.64) Z=0.24 0.814 ADA(U/L) 35.71(28.11~43.31) 28.02(20.73~35.31) Z=6.59 <0.001 APRI 0.39(0.19~0.51) 0.35(0.21~0.57) Z=1.35 0.172 AAR 0.94(0.49~1.39) 0.85(0.61~1.10) Z=1.31 0.193 FIB-4 0.81(0.05~1.58) 0.62(0.34~0.90) Z=1.87 0.064 表 2 LSM、SSM在观察者间的可重复性分析
指标 操作者A 操作者B P值1) 相关系数及P值 LSM(kPa) 20.81±1.82 21.32±1.97 0.305 0.75(P<0.001) SSM(kPa) 39.35±2.44 39.75±2.42 0.526 0.82(P<0.001) 注:1)采用配对样本t检验。 -
[1] TSENG Y, LI F, WANG J, et al. Spleen and liver stiffness for noninvasive assessment of portal hypertension in cirrhotic patients with large esophageal varices[J]. J Clin Ultrasound, 2018, 46(7): 442-449. DOI: 10.1002/jcu.22635. [2] CASTÉRA L, LE BAIL B, ROUDOT-THORAVAL F, et al. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: Comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores[J]. J Hepatol, 2009, 50(1): 59-68. DOI: 10.1016/j.jhep.2008.08.018. [3] BUECHTER M, KAHRAMAN A, MANKA P, et al. Spleen and liver stiffness is positively correlated with the risk of esophageal variceal bleeding[J]. Digestion, 2016, 94(3): 138-144. DOI: 10.1159/000450704. [4] STEFANESCU H, PROCOPET B, PLATON-LUPSOR M, et al. Is there any place for spleen stiffness measurement in portal hypertension?[J]. Am J Gastroenterol, 2013, 108(10): 1660-1661. DOI: 10.1038/ajg.2013.239. [5] SUN HY, WANG YJ, WANG M, et al. Liver biopsy results of chronic HBV carriers aged above 40 years: An analysis of 442 cases[J]. J Clin Hepatol, 2017, 33(8): 1479-1482. DOI: 10.3969/j.issn.1001-5256.2017.08.013.孙海英, 王雅君, 王明, 等. 442例40岁以上慢性HBV携带者肝穿刺活组织检查的结果分析[J]. 临床肝胆病杂志, 2017, 33(8): 1479-1482. DOI: 10.3969/j.issn.1001-5256.2017.08.013. [6] LI M, LI T, LIU ZJ, et al. Establishment and evaluation of a diagnostic modeI for liver fibrosis with the radio of serum markers as variables[J]. Shandong Med J, 2018, 58(18): 17-20. DOI: 10.3969/j.issn.1002-266X.2018.18.005.李曼, 李涛, 刘振杰, 等. 以血清学指标比值为变量的肝纤维化诊断模型构建与评价[J]. 山东医药, 2018, 58(18): 17-20. DOI: 10.3969/j.issn.1002-266X.2018.18.005. [7] KE YF, GAO GS, ZHU Z, et al. Value of serum adenosine deaminase in diagnosis of marked liver fibrosis in patients with chronic hepatitis B virus infection[J]. J Clin Hepatol, 2018, 34(4): 760-763. DOI: 10.3969/j.issn.1001-5256.2018.04.013.柯叶芳, 高国生, 朱喆, 等. 血清腺苷脱氨酶对慢性HBV感染者显著肝纤维化的诊断价值[J]. 临床肝胆病杂志, 2018, 34(4): 760-763. DOI: 10.3969/j.issn.1001-5256.2018.04.013. [8] KOTANI K, KAWABE J, MORIKAWA H, et al. Comprehensive screening of gene function and networks by DNA microarray analysis in Japanese patients with idiopathic portal hypertension[J]. Mediators Inflamm, 2015, 2015: 349215. DOI: 10.1155/2015/349215. [9] YANG YG, MA SH, LI FJ, et al. The clinical value of adenosine deaminase for diagnose of chronic viral hepatitis[J]. J Clin Hepatol, 2007, 23(1): 17-18. DOI: 10.3969/j.issn.1001-5256.2007.01.007.杨彦改, 马士恒, 李福建, 等. 腺苷脱氨酶在慢性病毒性肝病中的诊断价值研究[J]. 临床肝胆病杂志, 2007, 23(1): 17-18. DOI: 10.3969/j.issn.1001-5256.2007.01.007. [10] MA SH, YANG YG, CHANG YQ, et al. Study of the serum adenosine deaminase level in patients with hepatitis[J]. 2013, 30(1): 33-35. DOI: 10.3969/j.issn.1674-490X.2013.01.010.马士恒, 杨彦改, 常亚青, 等. 肝病患者血清腺苷脱氨酶检测的临床意义[J]. 医学研究与教育, 2013, 30(1): 33-35. DOI: 10.3969/j.issn.1674-490X.2013.01.010. [11] DENG H, QI X, GUO X. Diagnostic accuracy of APRI, AAR, FIB-4, FI, King, Lok, Forns, and FibroIndex Scores in predicting the presence of esophageal varices in liver cirrhosis: A systematic review and Meta-analysis[J]. Medicine (Baltimore), 2015, 94(42): e1795. DOI: 10.1097/MD.0000000000001795. [12] Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10. 3969 / j. issn. 1001 -5256. 2019. 12. 007.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10. 3969 / j. issn. 1001-5256. 2019. 12. 007. [13] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association; Chinese Society of Endoscopy, Chinese Medical Association. Guidelines for the diagnosis and treatment of esophageal and gastric variceal bleeding incirrhotic portalhy pertension[J]. J Clin Hepatol, 2016, 32(2): 203- 219. DOI: 10.3969/j.issn.1001-5256.2016.02.002.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会内镜学分会. 肝硬化门静脉高压食管胃静脉曲张出血的防治指南[J]. 临床肝胆病杂志, 2016, 32(2): 203-219. DOI: 10.3969/j.issn.1001-5256.2016.02.002. [14] ASRANI SK, DEVARBHAVI H, EATON J, et al. Burden of liver diseases in the world[J]. J Hepatol, 2019, 70(1): 151-171. DOI: 10.1016/j.jhep.2018.09.014. [15] KIM BK, HAN KH, PARK JY, et al. A liver stiffness measurement-based, noninvasive prediction model for high-risk esophageal varices in B-viral liver cirrhosis[J]. Am J Gastroenterol, 2010, 105(6): 1382-1390. DOI: 10.1038/ajg.2009.750. [16] GARCIA-TSAO G. Current management of the complications of cirrhosis and portal hypertension: Variceal hemorrhage, ascites, and spontaneous bacterial peritonitis[J]. Dig Dis, 2016, 34(4): 382-386. DOI: 10.1159/000444551. [17] GUO YL, LU XL, CHENG Y, et al. Combination measurement of liver and spleen stiffness with portal vein width to evaluate risk of bleeding in esophageal and gastric varices patients[J]. Chin J Hepatol, 2016, 24(1): 56-61. DOI: 10.3760/cma.j.issn.1007-3418.2016.01.011.郭芸蕾, 鲁晓岚, 程妍, 等. 肝脾硬度联合门静脉宽度评估肝硬化食管胃底静脉曲张出血风险[J]. 中华肝脏病杂志, 2016, 24(1): 56-61. DOI: 10.3760/cma.j.issn.1007-3418.2016.01.011. [18] YANG XP. Application value of liver and spleen stiffness detected by transient elastography for predicting esophageal varices[J]. Chin J Ultrasound Med, 2017, 33(2): 139-142. DOI: 10.3969/j.issn.1002-0101.2017.02.014.杨学平. 瞬时弹性成像检测肝脾硬度预测食管静脉曲张的价值[J]. 中国超声医学杂志, 2017, 33(2): 139-142. DOI: 10.3969/j.issn.1002-0101.2017.02.014. [19] FRAQUELLI M, GIUNTA M, POZZI R, et al. Feasibility and reproducibility of spleen transient elastography and its role in combination with liver transient elastography for predicting the severity of chronic viral hepatitis[J]. J Viral Hepat, 2014, 21(2): 90-98. DOI: 10.1111/jvh.12119. [20] SINGH S, EATON JE, MURAD MH, et al. Accuracy of spleen stiffness measurement in detection of esophageal varices in patients with chronic liver disease: Systematic review and meta-analysis[J]. Clin Gastroenterol Hepatol, 2014, 12(6): 935-945. e4. DOI: 10.1016/j.cgh.2013.09.013. [21] COLECCHIA A, COLLI A, CASAZZA G, et al. Spleen stiffness measurement can predict clinical complications in compensated HCV-related cirrhosis: A prospective study[J]. J Hepatol, 2014, 60(6): 1158-1164. DOI: 10.1016/j.jhep.2014.02.024. [22] BEN-SHOSHAN SO, KAGAN P, SULTAN M, et al. ADAR1 deletion induces NFκB and interferon signaling dependent liver inflammation and fibrosis[J]. RNA Biol, 2017, 14(5): 587-602. DOI: 10.1080/15476286.2016.1203501. [23] LI XK. Analysis of serum biochemical indexes in 117 patients with liver cirrhosis[J]. Chin J Lab Diagn, 2016, 20(10): 1673-1675. https://www.cnki.com.cn/Article/CJFDTOTAL-ZSZD201610020.htm李祥坤. 117例肝硬化患者血清多项生化指标的检测水平分析[J]. 中国实验诊断学, 2016, 20(10): 1673-1675. https://www.cnki.com.cn/Article/CJFDTOTAL-ZSZD201610020.htm [24] JIANG ZG, SANDHU B, FELDBRVGGE L, et al. Serum activity of macrophage-derived adenosine deaminase 2 is associated with liver fibrosis in nonalcoholic fatty liver disease[J]. Clin Gastroenterol Hepatol, 2018, 16(7): 1170-1172. DOI: 10.1016/j.cgh.2017.11.028. 期刊类型引用(2)
1. 中国抗癌协会肿瘤消融治疗专业委员会,中国临床肿瘤学会肿瘤消融治疗专家委员会,中国医师协会介入医师分会介入围手术学组. 复合式冷热消融治疗肝肿瘤围手术期管理专家共识. 中华内科杂志. 2025(02): 110-118 .  百度学术
百度学术2. 温钊,臧铁柱,宋晓改,张炫,江艳丽. 射频消融治疗包膜下与非包膜下早期肝细胞癌患者的远期预后. 河南医学研究. 2023(15): 2738-2744 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 2127 KB)
PDF下载 ( 2127 KB)


 下载:
下载:
 百度学术
百度学术

