HBV相关肝细胞癌患者血清N-糖组图谱改变与肝组织糖基转移酶表达变化的关系
DOI: 10.3969/j.issn.1001-5256.2021.06.024
Association between the alteration of serum N-glycan profile and the change of glycosyltransferase expression in liver tissue in patients with hepatitis B virus-related hepatocellular carcinoma
-
摘要:
目的 本研究通过对HBV相关肝细胞癌(HCC)患者血清N-聚糖检测及肝癌组织与癌旁组织中糖基转移酶基因表达水平比较分析,探索HCC患者血清N-聚糖变化的可能机制。 方法 收集解放军总医院2018年—2019年HBV相关HCC手术患者(34例)的肝癌和癌旁组织及正常肝组织标本,同时采集血清标本。从34例HCC患者中随机选择8例HCC患者血清标本作为HCC试验组,20例健康成年人血清标本作为对照组。应用DSA-FACE法分析HCC试验组与对照组血清N-聚糖图谱。采用荧光定量PCR法检测34例HBV相关HCC患者癌组织和癌旁组织中8种糖基转移酶基因(FUT3、FUT4、FUT6、FUT7、FUT8、Gn-TⅢ、Gn-TⅣa和Gn-TⅤ)mRNA表达水平,并应用蛋白印迹法检测相应蛋白表达水平。计量资料两组间比较采用独立样本t检验。 结果 与对照相比,8例HCC患者血清中N-聚糖峰9 (peak9, NA3Fb)的丰度明显升高(t=-2.514, P<0.05)。糖基转移酶FUT8、Gn-TⅣa和Gn-TⅤ基因mRNA表达水平在癌组织和癌旁组织间有差异,其中癌组织中FUT8和Gn-TⅤ基因的mRNA与蛋白表达水平显著高于癌旁组织(mRNA: 1.50±0.34 vs 0.65±0.11, t=-2.354,P=0.022; 3.57±0.64 vs 1.33±0.16, t=-3.384, P=0.001)(蛋白:0.70±0.11 vs 0.083±0.017, t=9.555,P=0.001; 1.33±0.19 vs 0.60±0.15, t=5.097, P=0.007);癌组织中Gn-TⅣa基因的mRNA表达水平显著高于癌旁组织(mRNA: 2.90±0.47 vs 1.68±0.19, t=-2.403,P=0.019),蛋白表达水平与癌旁组织无显著差异(蛋白:0.52±0.24 vs0.24±0.11, t=1.833, P=0.141)。癌组织中这些糖基转移酶表达改变与血清中N-聚糖丰度变化一致。 结论 HBV相关HCC患者血清中一些N-聚糖水平变化可能与肝癌组织中糖基转移酶基因GnT-V、GnT-IVa和FUT8表达上调密切相关。 Abstract:Objective To investigate the potential mechanism of serum N-glycan alterations in patients with hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) by measuring serum N-glycan profile and comparing glycosyltransferase gene expression between HCC tissue and adjacent tissue. Methods The samples of HCC tissue, adjacent tissue, and normal liver tissue were collected from 34 patients with HBV-related HCC who were admitted to Chinese PLA General Hospital, and serum samples were also collected. Among these 34 patients, 8 were randomly selected and their serum samples were established as HCC experimental group, and the serum samples of 20 healthy adults were established as control group. DNA sequencer-aided fluorophore-assisted carbohydrate electrophoresis was used to analyze serum N-glycan profile in the HCC experimental group and the control group. Quantitative real-time PCR was used to measure the mRNA expression of 8 glycosyltransferase genes (FUT3, FUT4, FUT6, FUT7, FUT8, Gn-TIII, Gn-TIVa, and Gn-TV) in the HCC tissue and adjacent tissue of 34 patients with HBV-related HCC, and Western blot was used to measure the expression of corresponding proteins. The independent samples t-test was used for comparison of continuous data between two groups. Results Compared with the control group, the HCC experimental group had a significant increase in the abundance of N-glycan peak9 (NA3Fb) in serum(t=-2.514, P < 0.05). There were significant differences in the mRNA expression of FUT8, Gn-TIVa, and Gn-TV between HCC tissue and adjacent tissue, and the mRNA and protein expression levels of FUT8 and Gn-TV in HCC tissue were significantly higher than those in adjacent tissue (FUT8 mRNA: 1.50±0.34 vs 0.65±0.11, t=-2.354, P=0.022; Gn-TV mRNA: 3.57±0.64 vs 1.33±0.16, t=-3.384, P=0.001; FUT8 protein: 0.70±0.11 vs 0.083±0.017, t=9.555, P=0.001; Gn-TV protein: 1.33±0.19 vs 0.60±0.15, t=5.097, P=0.007). The mRNA expression level of Gn-TIVa in HCC tissue was significantly higher than that in adjacent tissue (2.90±0.47 vs 1.68±0.19, t=-2.403, P=0.019), but there was no significant difference in the protein expression level of Gn-TIVa between HCC tissue and adjacent tissue (0.52±0.24 vs 0.24±0.11, t=1.833, P=0.141). The changes of glycosyltransferase gene expression in HCC tissue were consistent with the alteration of serum N-glycan profile. Conclusion Serum N-glycan alterations in patients with HBV-related HCC may be closely associated with the upregulated expression of the glycosyltransferase genes FUT8, Gn-TIVa, and Gn-TV in HCC tissue. -
Key words:
- Liver Neoplasms, Experimental /
- N-glycan /
- Glycosyltransferases
-
HBV慢性感染是肝细胞癌(HCC) 发生的最常见病因,HCC的发病率和病死率在恶性肿瘤中分别占第6位和第4位,严重威胁着人类健康[1]。HCC细胞异常增殖可使血清糖蛋白N-糖链组成和结构发生改变,因此,血清糖蛋白的N-聚糖可作为HCC诊断标志物和HCC治疗的分子靶点[2]。N-聚糖改变的基础在于肝细胞内糖基转移酶活性的变化,其中多数糖基转移酶表达与肝脏功能密切相关。当肝细胞发生癌变时,某些糖基转移酶基因被激活,其表达异常增加,或基因表达受抑制而含量下降,从而导致一些蛋白质异常糖基化修饰。大量研究[3]表明,这种异常糖基化修饰与肿瘤细胞恶性侵袭行为密不可分。笔者前期研究[4]发现,与健康对照相比,HBV相关HCC(HBV-HCC)患者血清中N-聚糖图谱发生一系列特征改变,其中二天线N-聚糖峰1(peak1, NGA2F)丰度升高;三天线N-聚糖峰9(peak9, NA3Fb)丰度特异性升高;其他三天线和四天线N-聚糖峰(peak10 NA3Fc、peak11 NA4、peak12 NA4Fb)丰度也有不同程度的升高。但目前HBV-HCC患者血清N-聚糖变化机制尚未完全阐明。本研究中,通过检测HBV-HCC患者癌组织与癌旁组织中8种重要的糖基转移酶基因(包括岩藻糖基转移酶FUT3、FUT4、FUT6、FUT7、FUT8和N-乙酰氨基葡萄糖转移酶Gn-TⅢ、Gn-TⅣa、Gn-TⅤ)表达水平变化并比较其差异,探索HBV-HCC患者血清N-聚糖变化的可能机制。
1. 材料与方法
1.1 肝组织标本和血清标本的收集
收集解放军总医院2018年9月—2019年11月HBV-HCC行手术患者的肝癌和癌旁组织及正常肝组织标本,同时采集血清标本,置于-80 ℃冰箱保存。患者符合以下入选标准: (1)均为感染HBV的HCC患者;(2)排除HAV、HCV、HDV、HEV等肝炎病毒感染;(3)患者术后病理标本均经医院病理科确诊为HCC,肝癌诊断符合原发性肝癌诊疗规范(2019年版)[5]。同时收集HCC患者临床资料。另外选取20例健康成年人血清作为对照。
1.2 DSA-FACE法检测HBV-HCC患者血清N-聚糖图谱
应用SPSS 20.0软件从34例HCC患者中随机选择8例HCC患者血清标本作为HCC试验组,20例健康成年人血清标本作为对照组。采用DSA-FACE法检测和分析血清N-聚糖图谱[6],具体步骤如下:
(1) 寡糖的释放:取3 μl血清,加入含有2 μl 10 mmol/L NH4HCO3缓冲液和3 μl去离子水的PCR反应板中, 反应板放入PCR仪器,95 ℃加热5 min后冷却至4 ℃, 然后加入3 μl PNGaseF(2.2 U/μl),37 ℃孵育3 h,之后加入100 μl去离子水终止反应,标记为D板。
(2) 标记寡糖:从D板吸取10 μl溶液加入一新的PCR反应板中,开盖在60 ℃条件下烘干90 min,加入3 μl标记溶液(20 mmol/L APTS∶ 1 mol/L NaCNBH3=1∶ 1),90 ℃反应2 h,加入100 μl去离子水终止反应,标记为L板。
(3) 去唾液酸:从L板中取2 μl溶液加入一新的PCR反应板,加入0.25 μL 100 mmol/L NH4Ac(pH=5.0)、0.2 μl唾液酸酶(2.5 U/μl)和1.55 μl去离子水, 震荡混匀后42 ℃孵育4 h,加40 μl去离子水终止反应,标记为DE板。
(4) DNA测序仪上机检测:取DE板10 μl溶液加入ABI测序仪专用96孔板,放入ABI 3500测序仪进行N-聚糖图谱分析,数据经GeneMapper软件分析。
1.3 荧光定量PCR法检测患者癌组织与癌旁组织中的糖基转移酶mRNA表达水平
冻存的肝组织放入超声震荡仪研磨后,用Trizol试剂提取总RNA,用Nano Drop One检测总RNA的浓度和纯度。将总RNA逆转录为cDNA后,用荧光定量PCR仪(ABI 7500 FAST)进行cDNA扩增,反应体系为20 μl。反应条件为:(1)95 ℃ 30 s;(2)95 ℃ 5 s,60 ℃ 34 s共40个循环;(3)溶解曲线:95 ℃ 15 s,60 ℃ 1 min, 95 ℃ 15 s。
以RPS11作为内参基因,目的基因的相对表达量用2-△△CT表示。癌组织和癌旁组织糖基转移酶mRNA相对表达量检测分析的对照均是正常肝组织。FUT3、FUT4、FUT6、FUT7、FUT8、Gn-TⅢ、Gn-TⅣa和Gn-TⅤ基因的特异引物由生工生物工程股份有限公司合成,引物序列见表 1。
表 1 荧光定量PCR检测糖基转移酶基因引物序列表基因 序列号 引物序列(5′→3′) FUT3 NM_001374740.1 F: CAA CAG AGA AAG CAG GCA
R: AAG AAA CAC ACA GCC ACC[7]FUT4 XM_032167182.1 F: TCC TAC GGA GAG GCT CAG
R: TCC TCG TAG TCC AAC ACG[7]FUT6 XM_011527879.3 F: CAT TTC TGC TGC CTC AGG
R: GGG CAA GTC AGG CAA CTC[7]FUT7 NM_004479.4 F: CCA CGA TCA CCA TCC TTG
R: AGG CTT CGG TTG GCA CTC[7]FUT8 XM_032180460.1 F: TCT AGC CGA GAA CTG TCC
R: GCT GCT CTT CTA AAA CGC[7]Gn-TⅢ XM_019018480.2 F: CCG CCA CAA GGT GCT CTA T
R: GAT CTC GTC CGC ATC GTC A[8]Gn-TⅣa XM_032178740.1 F: ACC AAG GGC ATA CGC TGG AG
R: GTT CTT GGT TGC CGC TAT GGA[9]Gn-TV XM_032411636.1 F: GCT GCC CAA CTG TAG GAG AC
R: GAA TCA AGG ACT CGG AGC AT[10]RPS11 XM_032159559.1 F: GCC GAG ACT ATC TGC ACT AC
R: ATG TCC AGC CTC AGA ACT TC[11]1.4 蛋白印迹法检测癌组织和癌旁组织中FUT8、Gn-TⅤ和Gn-Ⅳa的蛋白表达水平
冻存的肝组织放入超声震荡仪研磨后,用含cOmplete蛋白酶抑制剂的RIPA裂解液提取蛋白,用BCA蛋白试剂盒来测定蛋白浓度。电泳分离不同分子量蛋白后,15 V恒压下用半干转电转仪将蛋白转移至PVDF膜,于5%的脱脂奶粉中室温封闭1 h,加入抗-FUT8(1∶ 1000)、抗-Gn-TⅣa(1∶ 1000)、抗-Gn-TⅤ(1∶ 1000)、抗-β-actin(1∶ 4000)一抗,4 ℃过夜,TBST溶液洗膜3次,分别加入抗鼠或抗兔的二抗(1∶ 5000),室温下孵育2 h,TBST溶液洗膜3次,加入增强型ECL化学发光试剂,凝胶成像仪扫描显影的条带,ImageJ软件分析条带灰度。以β-actin作为内参,目的蛋白的相对表达量用目的蛋白灰度值与内参蛋白灰度值的比值来表示。
1.5 统计学方法
应用SPSS 20.0软件对数据进行统计分析。计量资料用x ±s表示,两组间比较采用独立样本t检验。所有统计学分析均采用双侧检验,P<0.05为差异具有统计学意义。
2. 结果
2.1 HBV-HCC患者临床特征
34例HBV-HCC患者临床特征见表 2。
表 2 34例HBV-HCC患者临床特征临床特征 数值 男性[例(%)] 29 (85.29) 年龄(岁) 54.85±8.20 HBV DNA(×106IU/ml) 2.35±7.15 AFP(ng/ml) 556.82±1995.22 ALT(U/L) 44.34±60.72 AST(U/L) 40.71±44.25 肿瘤直径(例) ≤5 cm 21 5~10 cm 9 ≥10 cm 4 2.2 HBV-HCC患者血清N-聚糖图谱特征
应用DSA-FACE法分析HCC试验组8例HBV-HCC患者与对照组20例健康成年人血清N-聚糖图谱(图 1),其特征改变与笔者前期研究发现的特征改变相同[4]。
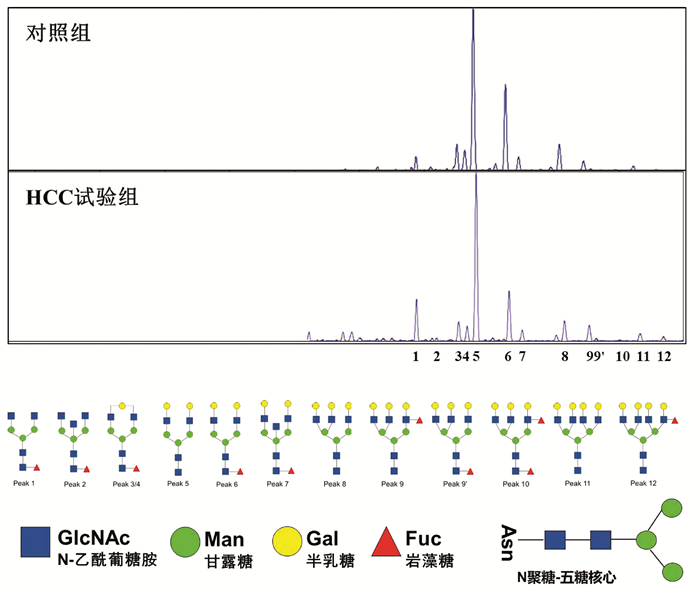
 图 1 HCC试验组与对照组血清N-聚糖图谱注:a,血清中12种N-聚糖丰度比较;b,12种N-聚糖结构。Peak1-Peak12图引自文献[4],Peak1: 二天线无半乳糖基核心ɑ-l, 6岩藻糖基化N聚糖(NGA2F);Peak2: 二天线无半乳糖基核心ɑ-l, 6岩藻糖基化平分型N聚糖(NGA2FB);Peak3/Peak4: 二天线核心ɑ-l, 6岩藻糖基化单支链半乳糖基N聚糖(NG1A2F);Peak5: 二天线N聚糖(NA2);Peak6: 二天线核心ɑ-l, 6岩藻糖基化N聚糖(NA2F);Peak7: 二天线平分型核心ɑ-l, 6岩藻糖基化N聚糖(NA2FB);Peak8: 三天线N聚糖(NA3);Peak9: 三天线支链ɑ-l, 3岩藻糖基化N聚糖(NA3Fb);Peak9’: 三天线核心ɑ-l, 6岩藻糖基化N聚糖(NA3Fc);Peak10: 三天线支链ɑ-l, 3岩藻糖基化与核心ɑ-l, 6岩藻糖基化N聚糖(NA3Fbc);Peak11: 四天线N聚糖(NA4);Peak12: 四天线支链ɑ-l, 3岩藻糖基化N聚糖(NA4 Fb)。
图 1 HCC试验组与对照组血清N-聚糖图谱注:a,血清中12种N-聚糖丰度比较;b,12种N-聚糖结构。Peak1-Peak12图引自文献[4],Peak1: 二天线无半乳糖基核心ɑ-l, 6岩藻糖基化N聚糖(NGA2F);Peak2: 二天线无半乳糖基核心ɑ-l, 6岩藻糖基化平分型N聚糖(NGA2FB);Peak3/Peak4: 二天线核心ɑ-l, 6岩藻糖基化单支链半乳糖基N聚糖(NG1A2F);Peak5: 二天线N聚糖(NA2);Peak6: 二天线核心ɑ-l, 6岩藻糖基化N聚糖(NA2F);Peak7: 二天线平分型核心ɑ-l, 6岩藻糖基化N聚糖(NA2FB);Peak8: 三天线N聚糖(NA3);Peak9: 三天线支链ɑ-l, 3岩藻糖基化N聚糖(NA3Fb);Peak9’: 三天线核心ɑ-l, 6岩藻糖基化N聚糖(NA3Fc);Peak10: 三天线支链ɑ-l, 3岩藻糖基化与核心ɑ-l, 6岩藻糖基化N聚糖(NA3Fbc);Peak11: 四天线N聚糖(NA4);Peak12: 四天线支链ɑ-l, 3岩藻糖基化N聚糖(NA4 Fb)。与对照组相比,HCC试验组患者三天线N-聚糖峰9(peak9,NA3Fb)丰度明显升高(t=-2.514,P<0.05);血清二天线N-聚糖峰1(peak1,NGA2F)和四天线N-聚糖峰(peak11 NA4、peak12 NA4Fb)的丰度在两组间差异无统计学意义(P值均>0.05)。
2.3 癌组织与癌旁组织8种糖基转移酶mRNA表达水平比较
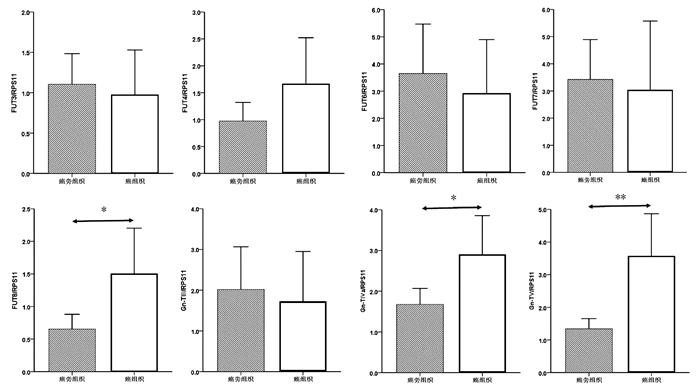
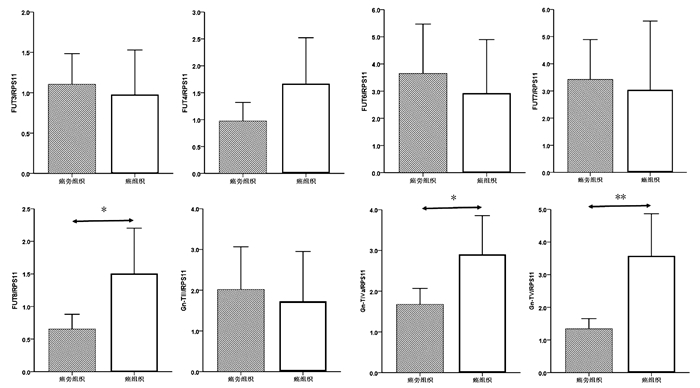
癌组织中FUT8、Gn-TⅣa和Gn-TⅤ基因mRNA表达水平显著高于癌旁组织(1.50±0.34 vs 0.65± 0.11, t=-2.354,P=0.022; 2.90±0.47 vs 1.68±0.19, t=-2.403,P=0.019; 3.57±0.64 vs 1.33±0.16, t=-3.384,P=0.001),差异均有统计学意义。FUT3、FUT4、FUT6、FUT7和Gn-TⅢ mRNA的表达水平在癌组织与癌旁组织间比较差异均无统计学意义(P值均>0.05)(图 2)。
进一步比较了HCC试验组中8例患者癌组织与癌旁组织中8种糖基转移酶mRNA表达水平。与癌旁组织相比,8例HCC试验组患者癌组织中Gn-TⅤ mRNA表达明显升高(Gn-TⅤ: 5.26±1.70 vs 1.49±0.33, t=-2.173, P=0.047);Gn-TⅢ、Gn-TⅣa、FUT4和FUT8 mRNA表达在癌组织与癌旁组织间无显著性差异(Gn-TⅢ: 1.03±0.46 vs 1.55±0.62, t=0.663, P=0.518;Gn-TⅣa: 5.15±1.50 vs 2.39±0.46, t=-1.752, P=0.102; FUT4: 1.56±1.12 vs 0.81±0.27, t=-0.653, P=0.524; FUT8: 2.61±1.26 vs 1.01±0.41, t=-1.213, P=0.245)。
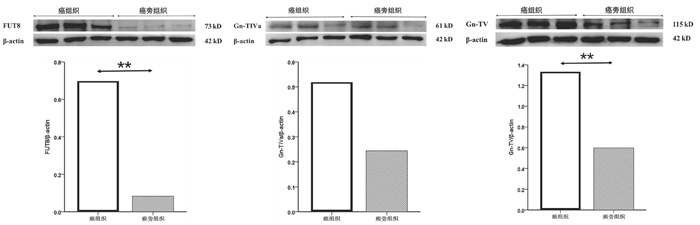
2.4 癌组织和癌旁组织FUT8、Gn-TⅣa和Gn-TⅤ基因的蛋白表达水平比较
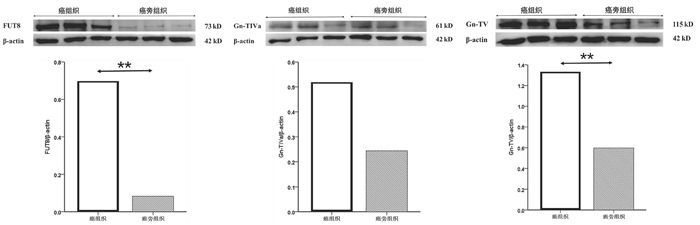
选取mRNA表达有显著性差异的3个糖基转移酶基因FUT8、Gn-TⅣa和Gn-TⅤ作为蛋白印迹实验的检测因子。结果如图 3所示,癌组织中FUT8与Gn-TⅤ的蛋白表达水平显著高于癌旁组织(0.70±0.11 vs 0.083±0.017, t=9.555, P=0.001; 1.33 ±0.19 vs 0.60±0.15, t=5.097, P=0.007);Gn-TⅣa的蛋白表达水平在癌组织与癌旁组织间比较差异无统计学意义(0.52±0.24 vs 0.24±0.11, t=1.833, P=0.141)。
3. 讨论
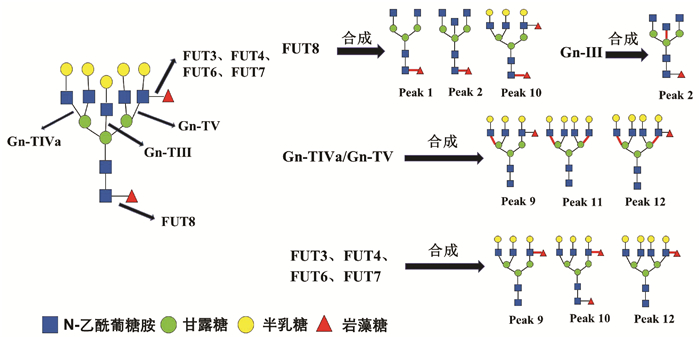
HCC患者血清中常出现大量异常糖基化N-糖蛋白,分离糖蛋白的N-聚糖链进行表达图谱分析,可发现支链与核心岩藻糖基化N-聚糖和多天线N-聚糖含量增加,这些N-聚糖与HCC发生发展密切相关,可作为筛查和诊断HCC的特异性标志[12]。研究[4, 13]发现在HBV-HCC患者血清中支链岩藻糖基化三天线N-聚糖(peak9, NA3Fb)丰度显著升高,且核心岩藻糖基化二天线N-聚糖(peak1, NGA2F)、核心岩藻糖基化平分型二天线N-聚糖(peak2, NGA2FB)、支链与核心岩藻糖基化三天线N聚糖(peak10, NA3Fc)、四天线N-聚糖(peak11, NA4)和支链岩藻糖基化四天线N-聚糖(peak12, NA4Fb)丰度也有不同程度的升高(N-聚糖结构如图 4)。本研究通过对HBV-HCC患者癌组织与癌旁组织相关糖基转移酶基因表达检测分析,探寻HCC患者特异性血清N-聚糖变化的可能机制。
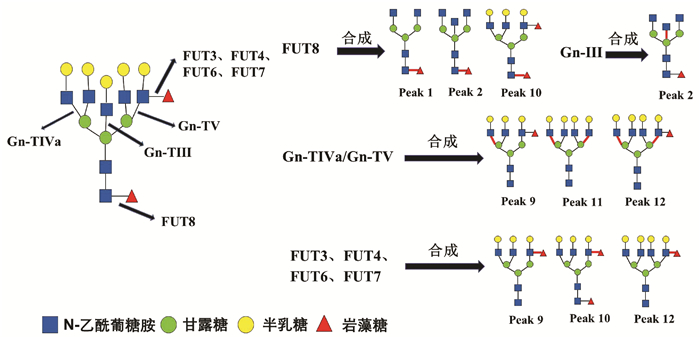
糖基转移酶Gn-TⅤ与Gn-TⅣa催化形成三天线及三天线以上N-聚糖的N-乙酰葡糖胺(GlcNAc)糖链结构[14-15],而Gn-TⅢ催化合成N-聚糖的平分型GlcNAc结构,Gn-TⅢ与Gn-TⅤ、Gn-TⅣa有拮抗作用(图 4)[16]。本研究发现,在癌组织中Gn-TⅤ与Gn-TⅣa mRNA表达水平显著高于癌旁组织(P<0.05),蛋白印迹实验也显示,在癌组织中Gn-TⅤ蛋白表达显著高于癌旁组织(P<0.05)。而Gn-TⅢ mRNA表达水平在癌组织与癌旁组织间无统计学差异(P=0.711)。本研究结果可以解释HCC试验组患者血清中N-聚糖变化:与对照组相比,8例HCC患者血清中三天线N-聚糖(peak9)丰度显著升高(P<0.05),而且这8例HCC患者癌组织Gn-TⅤ表达水平显著高于癌旁组织(P<0.05)。说明HCC患者血清中多天线N-聚糖丰度升高可能与糖基转移酶Gn-TⅤ高水平表达密切相关,促进含GlcNAc多分支(三天线及以上)N-聚糖的合成(图 4)。
以往研究[4, 13]表明,在HBV-HCC患者血清中核心岩藻糖基化N-聚糖(peak1、peak2、peak10)丰度较高。本研究发现,在癌组织中核心岩藻糖基转移酶FUT8 mRNA与蛋白表达水平显著高于癌旁组织(P<0.05)。FUT8表达上调会导致核心岩藻糖基化修饰结构的N-聚糖增加,可能与癌细胞高转移潜能有关[17]。另外,AFP是临床上最常用的HCC血清学检测指标,有研究[18]发现HCC患者血清中AFP经FUT8催化作用下可形成核心岩藻糖基化AFP(AFP-L3),其与肿瘤的发展速度、肿瘤大小和肿瘤转移密切相关,且诊断HCC效力优于AFP。除了α-1, 6核心岩藻糖基化N-聚糖,α-1, 3分支岩藻糖基化N-聚糖的丰度在HBV-HCC患者血清也特异性升高。α-1, 3分支岩藻糖基化结构是三天线N-聚糖(peak9)中Lewis X结构形成的关键[4],参与合成α-1, 3分支岩藻糖基化结构且与HCC密切相关的糖基转移酶有FUT3、FUT4、FUT6、FUT7[19]。本研究显示,在34例HCC患者癌组织与癌旁组织间FUT3、FUT4、FUT6和FUT7 mRNA表达水平没有显著性差异(P>0.05),不能解释血清N-聚糖水平异常改变。结合以往研究报道分析FUT基因表达的检测结果,可能因为多种岩藻糖基转移酶参与合成α-1, 3分支岩藻糖基化结构,而目前对于这些酶相互作用机制尚未明确,较难判断岩藻糖基转移酶中那一种亚类起主导作用。本研究主要检测8种与HCC密切相关的糖基转移酶基因表达,初步阐述HBV-HCC患者血清中N-聚糖变化的可能机制。但对于不同糖基转移酶之间相互作用的具体机制以及其他血清N-聚糖(如异常唾液酸化修饰的N-聚糖等)变化机制,还有待于进一步研究。
-
注:a,血清中12种N-聚糖丰度比较;b,12种N-聚糖结构。Peak1-Peak12图引自文献[4],Peak1: 二天线无半乳糖基核心ɑ-l, 6岩藻糖基化N聚糖(NGA2F);Peak2: 二天线无半乳糖基核心ɑ-l, 6岩藻糖基化平分型N聚糖(NGA2FB);Peak3/Peak4: 二天线核心ɑ-l, 6岩藻糖基化单支链半乳糖基N聚糖(NG1A2F);Peak5: 二天线N聚糖(NA2);Peak6: 二天线核心ɑ-l, 6岩藻糖基化N聚糖(NA2F);Peak7: 二天线平分型核心ɑ-l, 6岩藻糖基化N聚糖(NA2FB);Peak8: 三天线N聚糖(NA3);Peak9: 三天线支链ɑ-l, 3岩藻糖基化N聚糖(NA3Fb);Peak9’: 三天线核心ɑ-l, 6岩藻糖基化N聚糖(NA3Fc);Peak10: 三天线支链ɑ-l, 3岩藻糖基化与核心ɑ-l, 6岩藻糖基化N聚糖(NA3Fbc);Peak11: 四天线N聚糖(NA4);Peak12: 四天线支链ɑ-l, 3岩藻糖基化N聚糖(NA4 Fb)。
图 1 HCC试验组与对照组血清N-聚糖图谱
表 1 荧光定量PCR检测糖基转移酶基因引物序列表
基因 序列号 引物序列(5′→3′) FUT3 NM_001374740.1 F: CAA CAG AGA AAG CAG GCA
R: AAG AAA CAC ACA GCC ACC[7]FUT4 XM_032167182.1 F: TCC TAC GGA GAG GCT CAG
R: TCC TCG TAG TCC AAC ACG[7]FUT6 XM_011527879.3 F: CAT TTC TGC TGC CTC AGG
R: GGG CAA GTC AGG CAA CTC[7]FUT7 NM_004479.4 F: CCA CGA TCA CCA TCC TTG
R: AGG CTT CGG TTG GCA CTC[7]FUT8 XM_032180460.1 F: TCT AGC CGA GAA CTG TCC
R: GCT GCT CTT CTA AAA CGC[7]Gn-TⅢ XM_019018480.2 F: CCG CCA CAA GGT GCT CTA T
R: GAT CTC GTC CGC ATC GTC A[8]Gn-TⅣa XM_032178740.1 F: ACC AAG GGC ATA CGC TGG AG
R: GTT CTT GGT TGC CGC TAT GGA[9]Gn-TV XM_032411636.1 F: GCT GCC CAA CTG TAG GAG AC
R: GAA TCA AGG ACT CGG AGC AT[10]RPS11 XM_032159559.1 F: GCC GAG ACT ATC TGC ACT AC
R: ATG TCC AGC CTC AGA ACT TC[11]表 2 34例HBV-HCC患者临床特征
临床特征 数值 男性[例(%)] 29 (85.29) 年龄(岁) 54.85±8.20 HBV DNA(×106IU/ml) 2.35±7.15 AFP(ng/ml) 556.82±1995.22 ALT(U/L) 44.34±60.72 AST(U/L) 40.71±44.25 肿瘤直径(例) ≤5 cm 21 5~10 cm 9 ≥10 cm 4 -
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. [2] DRAKE RR. Glycosylation and cancer: Moving glycomics to the forefront[J]. Adv Cancer Res, 2015, 126: 1-10. DOI: 10.1016/bs.acr.2014.12.002. [3] HÄUSELMANN I, BORSIG L. Altered tumor-cell glycosylation promotes metastasis[J]. Front Oncol, 2014, 4: 28. DOI: 10.3389/fonc.2014.00028. [4] LIU XE, DESMYTER L, GAO CF, et al. N-glycomic changes in hepatocellular carcinoma patients with liver cirrhosis induced by hepatitis B virus[J]. Hepatology, 2007, 46(5): 1426-1435. DOI: 10.1002/hep.21855. [5] Bureau of Medical Administration, National Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China(2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007.中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗规范(2019年版)[J]. 临床肝胆病杂志, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. [6] CAO X, SHANG QH, CHI XL, et al. Serum N-glycan markers for diagnosing liver fibrosis induced by hepatitis B virus[J]. World J Gastroenterol, 2020, 26(10): 1067-1079. DOI: 10.3748/wjg.v26.i10.1067. [7] CHENG L, GAO S, SONG X, et al. Comprehensive N-glycan profiles of hepatocellular carcinoma reveal association of fucosylation with tumor progression and regulation of FUT8 by microRNAs[J]. Oncotarget, 2016, 7(38): 61199-61214. DOI: 10.18632/oncotarget.11284. [8] CHEN C, DIAO D, GUO L, et al. All-trans-retinoic acid modulates ICAM-1 N-glycan composition by influencing GnT-Ⅲ levels and inhibits cell adhesion and trans-endothelial migration[J]. PLoS One, 2012, 7(12): e52975. DOI: 10.1371/journal.pone.0052975. [9] NIE H, LIU X, ZHANG Y, et al. Specific N-glycans of Hepatocellular Carcinoma Cell Surface and the Abnormal Increase of Core-α-1, 6-fucosylated Triantennary Glycan via N-acetylglucosaminyltransferases-IVa Regulation[J]. Sci Rep, 2015, 5: 16007. DOI: 10.1038/srep16007. [10] WANG G, LIU J, CAI Y, et al. Loss of Barx1 promotes hepatocellular carcinoma metastasis through up-regulating MGAT5 and MMP9 expression and indicates poor prognosis[J]. Oncotarget, 2017, 8(42): 71867-71880. DOI: 10.18632/oncotarget.18288. [11] WU X, DAO THI VL, LIU P, et al. Pan-genotype hepatitis E virus replication in stem cell-derived hepatocellular systems[J]. Gastroenterology, 2018, 154(3): 663-674. e7. DOI: 10.1053/j.gastro.2017.10.041. [12] KIZUKA Y, TANIGUCHI N. Enzymes for N-glycan branching and their genetic and nongenetic regulation in cancer[J]. Biomolecules, 2016, 6(2). DOI: 10.3390/biom6020025. [13] FANG M, ZHAO YP, ZHOU FG, et al. N-glycan based models improve diagnostic efficacies in hepatitis B virus-related hepatocellular carcinoma[J]. Int J Cancer, 2010, 127(1): 148-159. DOI: 10.1002/ijc.25030. [14] CUI J, HUANG W, WU B, et al. N-glycosylation by N-acetylglucosaminyltransferase V enhances the interaction of CD147/basigin with integrin β1 and promotes HCC metastasis[J]. J Pathol, 2018, 245(1): 41-52. DOI: 10.1002/path.5054. [15] FAN J, WANG S, YU S, et al. N-acetylglucosaminyltransferase IVa regulates metastatic potential of mouse hepatocarcinoma cells through glycosylation of CD147[J]. Glycoconj J, 2012, 29(5-6): 323-334. DOI: 10.1007/s10719-012-9414-1. [16] ZHAO Y, NAKAGAWA T, ITOH S, et al. N-acetylglucosaminyltransferase Ⅲ antagonizes the effect of N-acetylglucosaminyltransferase V on alpha3beta1 integrin-mediated cell migration[J]. J Biol Chem, 2006, 281(43): 32122-32130. DOI: 10.1074/jbc.M607274200. [17] ZHAO Y, ITOH S, WANG X, et al. Deletion of core fucosylation on alpha3beta1 integrin down-regulates its functions[J]. J Biol Chem, 2006, 281(50): 38343-38350. DOI: 10.1074/jbc.M608764200. [18] DEBRUYNE EN, DELANGHE JR. Diagnosing and monitoring hepatocellular carcinoma with alpha-fetoprotein: New aspects and applications[J]. Clin Chim Acta, 2008, 395(1-2): 19-26. DOI: 10.1016/j.cca.2008.05.010. [19] KEELEY TS, YANG S, LAU E. The diverse contributions of fucose linkages in cancer[J]. Cancers (Basel), 2019, 11(9). DOI: 10.3390/cancers11091241. -




 PDF下载 ( 4153 KB)
PDF下载 ( 4153 KB)

 下载:
下载:




 下载:
下载: