枸杞多糖联合有氧运动对非酒精性脂肪肝炎大鼠模型的改善作用及其机制研究
DOI: 10.3969/j.issn.1001-5256.2021.06.026
Role and mechanism of Lycium barbarum polysaccharide combined with aerobic exercise in improving nonalcoholic steatohepatitis in rats
-
摘要:
目的 基于p38 MAPK/NF-κB途径探讨枸杞多糖联合有氧运动对高脂饮食诱导的非酒精性脂肪肝炎(NASH)大鼠肝脏的保护作用。 方法 8周龄SD大鼠共45只,适应性饲养1周后随机选取10只作为对照组给予普通饲料,剩余大鼠为高脂组(35只),食用高脂饲料。28周时将高脂组大鼠随机分为4组进行干预(每组8只),分别为模型组、枸杞多糖组、有氧运动组、联合组(枸杞多糖+有氧运动),干预周期为10周。实验结束时,测定所有大鼠空腹血糖,收集血清样本,取肝组织和内脏脂肪。生化试剂盒检测血清TG、TC、ALT、AST水平。ELISA试剂盒测定大鼠血清胰岛素(FINS)、TNFα、IL-6和单核细胞趋化蛋白-1(MCP-1)水平。实时定量PCR和Western Blot测定肝组织Toll样受体4(TLR4)、p38 MAPK和NF-κB的表达水平。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 与对照组相比,模型组大鼠TG、TC、AST、ALT、FINS和胰岛素抵抗指数(HOMA-IR)水平均显著升高(P值均<0.05),血清炎症因子MCP-1、TNFα和IL-6水平均呈现增高趋势(P值均<0.05),肝组织TLR4、p38 MAPK和NF-κB mRNA和蛋白表达水平均显著升高(P值均<0.05)。与模型组相比,各干预组大鼠TC、AST、ALT、FINS和HOMA-IR水平均显著降低(P值均<0.05),血清炎症因子MCP-1、TNFα和IL-6水平均呈现降低趋势(P值均<0.05);肝组织TLR4、p38 MAPK、NF-κB相关mRMA和蛋白水平均显著降低(P值均<0.05)。与枸杞多糖组相比, 联合组TG、ALT、FINS、HOMA-IR水平均显著降低(P值均<0.05),血清MCP-1水平呈现降低趋势(P<0.05),TLR4 mRNA相对表达水平显著降低(P<0.05),p38 MAPK、NF-κB蛋白水平均显著降低(P值均<0.05)。与有氧运动组相比,联合组FINS、HOMA-IR水平均显著降低(P值均<0.05),IL-6、MCP-1水平均显著降低(P值均<0.05),TLR4 mRNA相对表达水平显著降低(P值均<0.05)。 结论 枸杞多糖联合有氧运动可能通过调节p38 MAPK/NF-κB途径改善NASH大鼠炎症水平。 -
关键词:
- 非酒精性脂肪肝炎 /
- 枸杞多糖 /
- 运动 /
- 大鼠,Sprague-Dawley
Abstract:Objective To investigate the protective effect of Lycium barbarum polysaccharide (LBP) combined with aerobic exercise (AE) on the liver of rats with nonalcoholic steatohepatitis (NASH) induced by high-fat diet based on the p38 mitogen-activated protein kinase (p38 MAPK)-nuclear factor-κB (NF-κB) pathway. Methods After 1 week of adaptive feeding, 45 Sprague-Dawley rats, aged 8 weeks, were randomly divided into control group (10 rats fed with normal diet) and high-fat group (35 rats fed with high-fat diet). At the end of week 28, the high-fat group was randomly divided into model group, LBP group, AE group, and LBP+AE group, with 8 rats in each group, and intervention was performed for 10 weeks. At the end of the experiment, fasting blood glucose was measure for all rats, and serum samples, liver tissue, and visceral fat were collected. Biochemical kits were used to measure the serum levels of triglyceride (TG), total cholesterol (TC), alanine aminotransferase (ALT), and aspartate aminotransferase (AST); ELISA kits were used to measure the serum levels of fasting insulin (FINS), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and monocyte chemotactic protein-1 (MCP-1); quantitative real-time PCR and Western blot were used to measure the mRNA and protein expression levels of Toll-like receptor 4 (TLR4), p38 MAPK, and NF-κB in liver tissue. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results Compared with the control group, the model group had significant increases in TG, TC, AST, ALT, FINS, and homeostasis model assessment of insulin resistance (HOMA-IR) (all P < 0.05), a tendency of increases in the serum levels of the inflammatory factors MCP-1, TNF-α, and IL-6 (all P < 0.05), and significant increases in the mRNA and protein expression levels of TLR4, p38 MAPK, and NF-κB in liver tissue (all P < 0.05). Compared with the model group, each intervention group had significant reductions in TG, TC, AST, ALT, FINS, and HOMA-IR (all P < 0.05), a tendency of reductions in the serum levels of the inflammatory factors MCP-1, TNF-α, and IL-6 (all P < 0.05), and significant reductions in the mRNA and protein expression levels of TLR4, p38 MAPK, and NF-κB (all P < 0.05). Compared with LBP group, the LBP+AE group had significant reductions in TG, ALT, FINS, HOMA-IR, MCP-1, the mRNA expression level of TLR4, protein expression levels of p38 MAPK and NF-κB(all P < 0.05). Compared with Ae group, the LBP+ AE group had significant reductions in FINS, HOMA-IR, IL-6, MCP-1, the mRNA expression level of TLR4 (all P < 0.05). Conclusion LBP combined with AE may improve inflammation in NASH rats by regulating the p38 MAPK/NF-κB pathway. -
表 1 反转录聚合酶链反应引物序列
基因 引物序列(5′→3′) β-actin 上游:TGTCACCAACTGGGACGATA 下游:GGGGTGTTGAAGGTCTCAAA TLR4 上游:AAGTTATTGTGGTGGTGTCTAG 下游:GAGGTAGGTGTTTCTGCTAAG p38 MAPK 上游:AGAGTCTCTGTCGACCTGCT 下游:CATCAGGGTCGTGGTACTGAG NF-κB 上游:TGCATTCTGACCTTGCCTAT 下游:TCCAGTCTCCGAGTGAAGC 表 2 各组NASH大鼠体质量、肝湿重、内脏脂肪质量的比较
指标 对照组(n=8) 模型组(n=8) 枸杞多糖组(n=8) 有氧运动组(n=8) 联合组(n=8) F值 P值 体质量(g) 625.6±36.0 832.4±57.81) 809.35±63.83 731.96±67.162) 719.64±58.502)3) 17.17 <0.05 肝湿质量(g) 15.18±1.62 16.62±1.301) 17.38±1.30 15.32±2.362) 14.55±1.062)3) 6.12 <0.05 内脏脂肪质量(g) 21.66±9.94 84.63±19.151) 80.02±11.70 58.38±21.102) 56.89±15.302)3) 20.45 <0.05 注: 与对照组相比,1) P<0.05;与模型组相比,2) P<0.05;与枸杞多糖组相比,3) P<0.05。 表 3 各组NASH大鼠NAS积分的比较
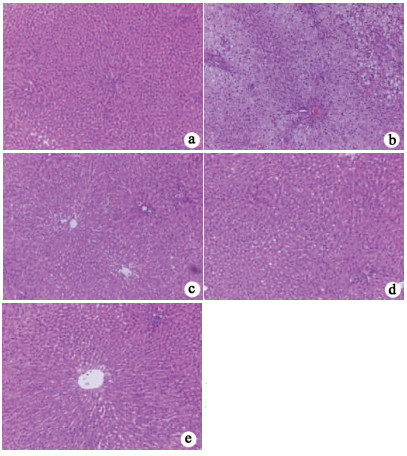
组别 动物数(只) NAS积分 对照组 8 0.67±0.58 模型组 8 6.67±1.531) 枸杞多糖组 8 3.67±0.582) 有氧运动组 8 3.33±0.582) 联合组 8 2.33±0.582) F值 19.77 P值 <0.05 注:与对照组相比,1) P<0.05;与模型组相比,2) P<0.05。 表 4 各组大鼠血脂、肝功能指标、FINS和HOMA-IR的比较
指标 对照组(n=8) 模型组(n=8) 枸杞多糖组(n=8) 有氧运动组(n=8) 联合组(n=8) F值 P值 TG(mmol/L) 1.17±0.25 1.55±0.501) 1.37±0.18 0.94±0.262) 0.88±0.152)3) 6.70 <0.05 TC(mmol/L) 3.84±0.50 4.93±0.981) 3.52±0.802) 3.52±0.472) 3.29±0.752) 5.05 <0.05 AST(U/L) 28.51±2.78 35.07±2.571) 28.23±3.232) 27.33±5.352) 24.78±4.632) 7.99 <0.05 ALT(U/L) 32.48±6.70 55.29±16.591) 32.94±8.592) 23.13±9.662) 20.74±7.962)3) 6.68 <0.05 FINS(mg/dl) 1.15±0.53 3.85±0.551) 2.21±0.822) 2.29±0.752) 1.40±0.822)3)4) 22.58 <0.05 HOMA-IR 5.80±2.87 24.65±10.591) 13.78±4.662) 16.06±5.472) 8.68±3.422)3)4) 11.33 <0.05 注:与对照组相比,1)P<0.05;与模型组相比,2)P<0.05;与枸杞多糖组相比,3)P<0.05;与有氧运动组相比,4)P<0.05。 表 5 各组大鼠血清TNFα、IL-6和MCP-1水平的比较
指标 对照组(n=8) 模型组(n=8) 枸杞多糖组(n=8) 有氧运动组(n=8) 联合组(n=8) F值 P值 TNFα(pg/ml) 54.14±12.48 234.57±55.011) 157.05±24.162) 166.96±33.022) 115.33±52.172) 18.28 <0.05 IL-6(pg/ml) 86.84±12.37 315.71±42.001) 133.63±10.132) 159.75±20.642) 129.27±24.012)4) 77.23 <0.05 MCP-1(pg/ml) 4.63±0.37 6.01±1.021) 3.91±1.372) 3.58±1.032) 2.37±0.402)3)4) 15.13 <0.05 注:与对照组相比,1)P<0.05;与模型组相比,2)P<0.05;与枸杞多糖组相比,3)P<0.05;与有氧运动组相比,4)P<0.05。 表 6 各组大鼠肝组织TLR4、p38 MAPK和NF-κB mRMA表达水平的比较
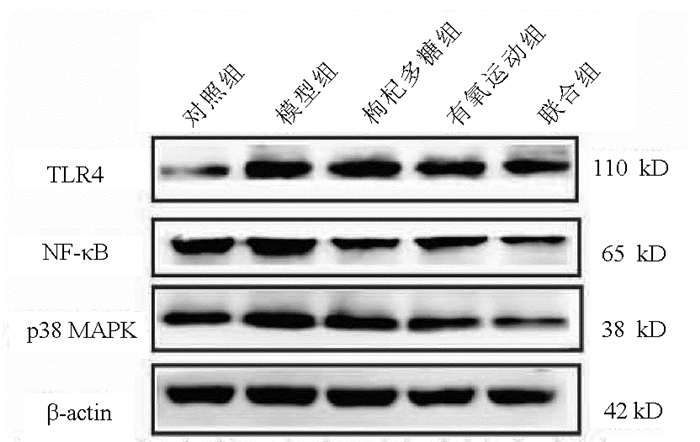
指标 对照组(n=8) 模型组(n=8) 枸杞多糖组(n=8) 有氧运动组(n=8) 联合组(n=8) F值 P值 TLR4mRNA 1.10±0.24 2.52±0.441) 1.85±0.642) 1.41±0.572) 0.60±0.142)3)4) 20.69 <0.05 p38MAPKmRNA 1.06±0.06 2.47±0.401) 2.00±0.492) 1.87±0.432) 1.81±0.132) 10.07 <0.05 NF-κBmRNA 1.22±0.22 1.58±0.191) 1.33±0.202) 1.31±0.112) 1.29±0.112) 3.27 0.03 注:与对照组相比,1)P<0.05;与模型组相比,2)P<0.05;与枸杞多糖组相比,3)P<0.05;与有氧运动组相比,4)P<0.05。 表 7 组大鼠肝组织TLR4、p38 MAPK和NF-κB蛋白表达水平的比较
指标 对照组(n=8) 模型组(n=8) 枸杞多糖组(n=8) 有氧运动组(n=8) 联合组(n=8) F值 P值 TLR4 0.53±0.06 0.86±0.091) 0.64±0.162) 0.60±0.132) 0.49±0.082) 5.25 0.02 p38MAPK 0.81±0.06 1.18±0.051) 0.90±0.062) 0.86±0.042) 0.78±0.052)3) 35.42 <0.05 NF-κB 0.75±0.05 1.12±0.101) 0.90±0.052) 0.80±0.162) 0.71±0.082)3) 8.43 <0.05 注:与对照组相比,1)P<0.05;与模型组相比,2)P<0.05;与枸杞多糖组相比,3)P<0.05。 -
[1] LU XL, JIANG YY, CAO Q. The role of oxidative stress and nuclear factor erythroid 2-related factor 2 in nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2020, 36(4): 924-927. DOI: 10.3969/j.issn.1001-5256.2020.04.048.陆孝良, 蒋元烨, 曹勤. 氧化应激与核因子E2相关因子2在非酒精性脂肪性肝病中的作用[J]. 临床肝胆病杂志, 2020, 36(4): 924-927. DOI: 10.3969/j.issn.1001-5256.2020.04.048. [2] BOECKMANS J, NATALE A, ROMBAUT M, et al. Flow cytometric quantification of neutral lipids in a human skin stem cell-derived model of NASH[J]. MethodsX, 2020, 7: 101068. DOI: 10.1016/j.mex.2020.101068. [3] NSEIR W, MAHAMID M. Statins in nonalcoholic fatty liver disease and steatohepatitis: Updated review[J]. Curr Atheroscler Rep, 2013, 15(3): 305. DOI: 10.1007/s11883-012-0305-5. [4] XIAO J, LIONG EC, CHING YP, et al. Lycium barbarum polysaccharides protect rat liver from non-alcoholic steatohepatitis-induced injury[J]. Nutr Diabetes, 2013, 3: e81. DOI: 10.1038/nutd.2013.22. [5] JIA L, LI W, LI J, et al. Lycium barbarum polysaccharide attenuates high-fat diet-induced hepatic steatosis by up-regulating SIRT1 expression and deacetylase activity[J]. Sci Rep, 2016, 6: 36209. DOI: 10.1038/srep36209. [6] XIAO J, XING F, HUO J, et al. Lycium barbarum polysaccharides therapeutically improve hepatic functions in non-alcoholic steatohepatitis rats and cellular steatosis model[J]. Sci Rep, 2014, 4: 5587. DOI: 10.1038/srep05587. [7] TAKAHASHI H, KOTANI K, TANAKA K, et al. Therapeutic approaches to nonalcoholic fatty liver disease: Exercise intervention and related mechanisms[J]. Front Endocrinol (Lausanne), 2018, 9: 588. DOI: 10.3389/fendo.2018.00588. [8] GUO YQ, WU Q, WU YT, et al. Effect of Lycium barbarum polysaccharide and aerobic exercise on rats with non-alcoholic fatty liver disease and its mechanism[J]. J Shanghai Jiaotong Univ(Med Sci), 2020, 40(1): 30-36. DOI: 10.3969/j.issn.1674-8115.2020.01.005.郭怡琼, 吴琼, 吴雅婷, 等. 枸杞多糖和有氧运动对大鼠非酒精性脂肪肝的干预效果及其机制研究[J]. 上海交通大学学报(医学版), 2020, 40(1): 30-36. DOI: 10.3969/j.issn.1674-8115. 2020.01.005. [9] NAKAMURA A, TERAUCHI Y. Lessons from mouse models of high-fat diet-induced NAFLD[J]. Int J Mol Sci, 2013, 14(11): 21240-21257. DOI: 10.3390/ijms141121240. [10] GONG XW, XU YJ, YANG QH, et al. Effect of soothing gan (liver) and invigorating pi (spleen) recipes on TLR4-p38 MAPK pathway in kupffer cells of non-alcoholic steatohepatitis rats[J]. Chin J Integr Med, 2019, 25(3): 216-224. DOI: 10.1007/s11655-018-2829-6. [11] The Chinese National Workshop on Fatty Liver and Alcoholic Liver Disease for the Chinese Liver Disease Association. Guidelines for management of nonalcoholic fatty liver disease: an updated and revised edition[J]. J Clin Hepatol, 2010, 26 (2): 120-124. http://lcgdbzz.org/cn/article/doi/1001-5256%20(2010)%2002-0120-05中华医学会肝病学分会脂肪肝和酒精性肝病学组. 非酒精性脂肪性肝病诊疗指南[J]. 临床肝胆病杂志, 2010, 26 (2): 120-124. http://lcgdbzz.org/cn/article/doi/1001-5256%20(2010)%2002-0120-05 [12] YIMIN, FURUMAKI H, MATSUOKA S, et al. A novel murine model for non-alcoholic steatohepatitis developed by combination of a high-fat diet and oxidized low-density lipoprotein[J]. Lab Invest, 2012, 92(2): 265-281. DOI: 10.1038/labinvest.2011.159. [13] ESTES C, RAZAVI H, LOOMBA R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease[J]. Hepatology, 2018, 67(1): 123-133. DOI: 10.1002/hep.29466. [14] ESTES C, ANSTEE QM, ARIAS-LOSTE MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030[J]. J Hepatol, 2018, 69(4): 896-904. DOI: 10.1016/j.jhep.2018.05.036. [15] BENEDICT M, ZHANG X. Non-alcoholic fatty liver disease: An expanded review[J]. World J Hepatol, 2017, 9(16): 715-732. DOI: 10.4254/wjh.v9.i16.715. [16] DENG Y, TANG K, CHEN R, et al. Effects of Shugan-Jianpi recipe on the expression of the p38 MAPK/NF-κB signaling pathway in the hepatocytes of NAFLD rats[J]. Medicines (Basel), 2018, 5(3). DOI: 10.3390/medicines5030106. [17] RECTOR RS, UPTERGROVE GM, MORRIS EM, et al. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model[J]. Am J Physiol Gastrointest Liver Physiol, 2011, 300(5): g874-g883. DOI: 10.1152/ajpgi.00510.2010. [18] YANG QH, XU YJ, LIU YZ, et al. Effects of Chaihu-Shugan-San and Shen-Ling-Bai-Zhu-San on p38 MAPK pathway in Kupffer cells of nonalcoholic steatohepatitis[J]. Evid Based Complement Alternat Med, 2014, 2014: 671013. DOI: 10.1155/2014/671013. [19] DONOHOE F, WILKINSON M, BAXTER E, et al. Mitogen-activated protein kinase (MAPK) and obesity-related cancer[J]. Int J Mol Sci, 2020, 21(4). DOI: 10.3390/ijms21041241. [20] LUEDDE T, SCHWABE R F. NF-κB in the liver-linking injury, fibrosis and hepatocellular carcinoma[J]. Nat Rev Gastro Hepat, 2011, 8(2): 108-118. DOI: 10.1038/nrgastro.2010.213 [21] KANG HH, KIM IK, LEE HI, et al. Chronic intermittent hypoxia induces liver fibrosis in mice with diet-induced obesity via TLR4/MyD88/MAPK/NF-κB signaling pathways[J]. Biochem Bioph Res Co, 2017, 490(2): 349-355. DOI: 10.1016/j.bbrc.2017.06.047. [22] WANG XA, ZHANG R, SHE ZG, et al. Interferon regulatory factor 3 constrains IKKβ/NF-κB signaling to alleviate hepatic steatosis and insulin resistance[J]. Hepatology, 2014, 59(3): 870-885. DOI: 10.1002/hep.26751. [23] LEE DE, LEE SJ, KIM SJ, et al. Curcumin ameliorates nonalcoholic fatty liver disease through inhibition of O-GlcNAcylation[J]. Nutrients, 2019, 11(11). DOI: 10.3390/nu11112702. [24] MANNE V, HANDA P, KOWDLEY KV. Pathophysiology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis[J]. Clin Liver Dis, 2018, 22(1): 23-37. DOI: 10.1016/j.cld.2017.08.007. -



 PDF下载 ( 2085 KB)
PDF下载 ( 2085 KB)

 下载:
下载: