消退素D1预处理对肝缺血再灌注损伤大鼠模型的保护作用及机制
DOI: 10.3969/j.issn.1001-5256.2021.06.030
Protective effect of resolvin D1 pretreatment in a rat model of hepatic ischemia-reperfusion injury and its mechanism
-
摘要:
目的 探究消退素D1(RvD1)对肝缺血再灌注(IR)损伤大鼠模型的保护作用及与血红素氧合酶-1(HO-1)之间的关系。 方法 Sprague-Dawley大鼠36只随机分为6组,分别为假手术(sham)+PBS组、sham+RvD1高剂量(10 μg/kg)组、IR+PBS组、IR+RvD1(2 μg/kg)低剂量组、IR+RvD1(5 μg/kg)中剂量组和IR+RvD1(10 μg/kg)高剂量组,每组6只。RvD1于缺血前1 h腹腔注射。生化仪测定ALT、AST水平,酶联免疫法检测血浆TNFα、IL-6、IL-8水平,HE染色观察肝组织学变化,Western Blot方法检测肝组织HO-1变化。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 与IR+PBS组相比,IR+RvD1中剂量组和IR+RvD1高剂量组大鼠ALT、AST水平以及炎症因子TNFα、IL-6、IL-8水平均明显降低(P值均<0.05),且中、高剂量两组间比较差异均无统计学意义(P值均>0.05)。Western Blot结果显示IR+RvD1中剂量组和IR+RvD1高剂量组肝脏HO-1蛋白表达较IR+PBS组升高(P值均<0.05)。HE染色观察肝组织学变化显示,与IR+PBS组相比,IR+RvD1中剂量组和IR+RvD1高剂量组细胞肿胀及肝索排列紊乱依然存在,但未见明显大片坏死区域。 结论 RvD1可能通过增加肝脏HO-1表达,降低炎症因子(TNFα、IL-6、IL-8)和转氨酶(ALT、AST)水平,发挥对大鼠肝脏IR损伤的保护作用。 -
关键词:
- 肝疾病 /
- 再灌注损伤 /
- 消退素D1 /
- 大鼠, Sprague-Dawley
Abstract:Objective To investigate the protective effect of resolvin D1 (RvD1) in a rat model of hepatic ischemia/reperfusion (IR) injury and its association with heme oxygenase-1 (HO-1). Methods A total of 36 Sprague-Dawley rats were randomly divided into sham-operation (sham)+phosphate-buffered saline (PBS) group, sham+high-dose RvD1 (10 μg/kg) group, IR+PBS group, IR+low-dose RvD1 (2 μg/kg) group, IR+middle-dose RvD1 (5 μg/kg) group, and IR+high-dose RvD1 (10 μg/kg) group, with 6 rats in each group. RvD1 were intraperitoneally injected at 1 hour before ischemia. A biochemical analyzer was used to measure the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST); ELISA was used to measure the plasma levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-8 (IL-8); HE staining was used to observe the histological changes of the liver; Western blot was used to measure the change in HO-1 in liver tissue. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results Compared with the IR+PBS group, the IR+middle-dose RvD1 group and the IR+high-dose RvD1 group had significant reductions in the levels of ALT, AST, and the inflammatory factors TNF-α, IL-6, and IL-8 (all P < 0.05), and there were no significant differences between the IR+middle-dose RvD1 group and the IR+high-dose RvD1 group (all P > 0.05). Western blot showed that compared with the IR+PBS group, the IR+middle-dose RvD1 group and the IR+high-dose RvD1 group had a significant increase in the protein expression of HO-1 in the liver (P < 0.05). HE staining showed that compared with the IR+PBS group, the IR+middle-dose RvD1 group and the IR+high-dose RvD1 group had cell swelling and disordered hepatic cords, without massive necrosis. Conclusion RvD1 exerts a protective effect against hepatic IR injury in rats by increasing the expression of HO-1 and reducing the levels of inflammatory factors (TNF-α, IL-6, and IL-8) and aminotransferases (ALT and AST). -
Key words:
- Liver Diseases /
- Reperfusion Injury /
- Resolvin D1 /
- Rats, Sprague-Dawley
-
肝缺血再灌注(ischemia reperfusion, IR)损伤是因休克、移植、部分肝脏切除后再灌注而出现的一系列肝细胞结构损伤和功能障碍。其发生的病理生理机制复杂,有诸多因素参与,包括炎性小体[1]、活性氧(ROS)产生、细胞内钙超载、核因子-κB激活、Kupffer细胞激活、细胞因子释放、黏附分子表达、中性粒细胞聚集、微循环障碍、线粒体功能障碍[2]等。消退素是一种内源性促炎症介质消退的介质,分为3种类型,即消退素E(resolvin E, RvE)、消退素D(resolvin D, RvD)、阿司匹林触发的消退素D(aspirin triggered resolvin D, AT-RvD)[3]。3种消退素均来源于ω-3多不饱和脂肪酸[4],其中RvE来源于二十碳五烯酸(eicosapentaenoic acid, EPA),而RvD与AT-RvD则来源于二十二碳六烯酸(docosahexaenoic acid, DHA)。RvD具有明显的促进炎症消退、保护细胞的作用[5],根据其羟基及双键的不同,又分为4型,即RvD1~4。RvD1对急性肺损伤[6]、脂肪性肝炎、糖尿病、帕金森氏病[7]等多种疾病具有保护作用。血红素氧合酶(heme oxygenase, HO) 是催化血红素转化为胆绿素的限速酶,同时产生一氧化碳和游离铁离子。HO有3种同工酶,其中HO-1又称为热休克蛋白-32,在应激性肝脏损伤,尤其是IR中发挥重要保护作用[8]。因此,本研究利用酶联免疫、HE染色和Western Blot等技术,检测在肝脏IR过程中RvD1发挥的作用,并初步探讨其保护机制与HO-1的关系,以期为临床相关疾病的治疗提供思路。
1. 材料与方法
1.1 实验动物
清洁级雄性Sprague-Dawley大鼠36只,购自北京维通利华实验动物技术有限公司,生产许可证编号:SCXK(京)2016-0011,使用许可证编号:SYXK(冀)2017-002,体质量250~300 g,实验动物正常饲养。
1.2 仪器和试剂
生化自动分析仪(购自德国Bayer公司),实时定量PCR仪(购自美国Thermo公司),LG16-W离心机(湖南湘仪实验室开发仪器有限公司)。RvD1购自Santa Cruz Biotechnology。大鼠血浆TNFα、IL-6、IL-8的酶联免疫试剂盒购自Solarbio公司。
1.3 实验方法
1.3.1 动物分组
36只SD大鼠随机分为6组:假手术(sham)+PBS组、sham+RvD1高剂量(10 μg/kg)组、IR+PBS组、IR+RvD1(2 μg/kg)低剂量组、IR+RvD1(5 μg/kg)中剂量组和IR+RvD1(10 μg/kg)高剂量组。手术前12 h禁食,3 h禁水。以1%戊巴比妥钠40 mg/kg腹腔注射麻醉大鼠。参考既往动物实验[9]制造肝脏约70%缺血模型,无菌条件下腹部正中切口约2 cm至剑突下,暴露肝脏,分离供应肝左叶和中叶的肝动脉和门静脉,用显微血管夹夹闭两者,造成肝脏约70%缺血模型,缺血45 min后松开血管夹,分层缝合关腹部。再灌注8 h后,腹腔注射麻醉,大鼠下腔静脉取血,离心取上清冻存。摘取肝脏,部分迅速置入4%多聚甲醛固定,部分置入Ep管冻存。具体情况如下:(1)sham+PBS组(n=6),给予腹腔注射PBS 1 ml,1 h后麻醉做腹部正中切口,缝合关闭;(2)sham+RvD1高剂量组(n=6),给予腹腔注射RvD1(10 μg/kg),1 h后麻醉做腹部正中切口,缝合关闭;(3)IR+PBS组(n=6),腹腔注射PBS 1 ml,1 h后腹部正中切口,暴露肝脏,分离供应肝左叶和中叶的肝动脉和门静脉,用显微血管夹夹闭二者,钳夹约45 min后,松开血管夹,分层缝合关腹。再灌注8 h后麻醉处死;(4)IR+RvD1低剂量组(n=6),缺血前1 h进行腹腔注射RvD12 μg/kg,余操作同IR+PBS组;(5)IR+RvD1中剂量组(n=6),缺血前1 h进行腹腔注射RvD1 5 μg/kg,余操作同IR+PBS组;(6)IR+RvD1高剂量组(n=6),缺血前1 h进行腹腔注射RvD1 10 μg/kg,余操作同IR+PBS组。具体详见表 1。
表 1 实验动物分组组别 过程 缺血前1 h(腹腔注射) 缺血 缺血45 min后再灌注 再灌注8 h sham+PBS组 PBS 1 ml 开关腹 取标本 sham+RvD1高剂量组 RvD1 10 μg/kg 开关腹 取标本 IR+PBS组 PBS 1 ml 钳夹缺血 再灌注,关腹 取标本 IR+RvD1低剂量组 RvD1 2 μg/kg 钳夹缺血 再灌注,关腹 取标本 IR+RvD1中剂量组 RvD1 5 μg/kg 钳夹缺血 再灌注,关腹 取标本 IR+RvD1高剂量组 RvD1 10 μg/kg 钳夹缺血 再灌注,关腹 取标本 1.3.2 血浆转氨酶检测
采集大鼠下腔静脉血,分离血浆,利用生化仪检测ALT、AST水平。
1.3.3 HE染色观察各组大鼠肝损伤情况
再灌注8 h后取大鼠肝脏固定于4%多聚甲醛,包埋切片染色,观察组织细胞学变化。
1.3.4 酶联免疫法检测各组大鼠血浆TNFα、IL-6、IL-8水平变化
各组大鼠血浆离心取上清,-80 ℃冻存备用。
1.3.5 Western Blot方法检测各组大鼠肝脏组织中HO-1水平变化
各组大鼠麻醉处死后留取肝脏组织,-80 ℃冻存备用。提取肝组织总蛋白,BCA法检测蛋白含量,蛋白变性、电泳及转膜、免疫杂交、显影,扫描。
1.4 伦理学审查
本研究方案经由河北大学实验动物伦理审查委员会审批,批号:2019001XB,符合实验室动物管理与使用准则。
1.5 统计学方法
采用SPSS 24.0统计软件包进行数据处理。计量资料以x±s表示,多组间比较采用单因素方差分析,组间进一步两两比较采用LSD-t检验。P<0.05为差异有统计学意义。
2. 结果
2.1 不同剂量RvD1对IR大鼠肝脏转氨酶的影响
再灌注8 h后,IR+PBS组与sham+PBS组相比,血浆ALT、AST水平明显升高(P值均<0.05);与IR+PBS组相比,IR+RvD1中剂量组和IR+RvD1高剂量组ALT、AST水平明显降低(P值均<0.05),而中、高剂量组间差异均无统计学意义(P值均>0.05)(表 2)。
表 2 各组大鼠血浆转氨酶水平比较组别 动物数(只) ALT(U/L) AST(U/L) sham+PBS组 6 56.88±11.65 58.45±12.11 sham+RvD1高剂量组 6 56.98±14.56 49.78±15.04 IR+PBS组 6 498.68±111.581) 606.35±150.061) IR+RvD1低剂量组 6 578.23±89.50 610.92±77.30 IR+RvD1中剂量组 6 373.13±105.592) 350.72±95.422) IR+RvD1高剂量组 6 348.17±86.522) 369.72±94.252) F值 43.926 47.459 P值 <0.001 <0.001 注:与sham+PBS组比较,1)P<0.05;与IR+PBS组比较,2)P<0.05。 2.2 不同剂量RvD1对IR大鼠肝组织损伤的影响
HE染色结果显示,IR+PBS组与sham+PBS组相比,肝细胞肿胀明显,肝索排列紊乱,大量单核细胞浸润,可见大片坏死区域;IR+RvD1中剂量组和IR+RvD1高剂量组细胞肿胀及肝索排列紊乱亦存在,但未见明显大片坏死区域。sham+RvD1高剂量组与sham+PBS组相比,肝细胞未见明显变化,肝索排列规律,中央静脉位置正常(图 1)。
2.3 不同剂量RvD1对IR大鼠血浆炎症因子TNFα、IL-6、IL-8的影响
再灌注8 h后,IR+PBS组与sham+PBS相比,血浆TNFα、IL-6、IL-8水平明显升高(P值均<0.05);与IR+PBS组相比,IR+RvD1中剂量组和IR+RvD1高剂量组的TNFα、IL-6、IL-8水平均明显降低(P值均<0.05),但中、高剂量两组之间差异均无统计学意义(P值均>0.05)(表 3)。
表 3 各组大鼠血浆炎症因子TNFα、IL-6、IL-8水平比较组别 动物数(只) IL-6(pg/ml) IL-8(pg/ml) TNFα(pg/ml) sham+PBS组 6 26.32±7.05 120.83±19.17 37.30±6.59 sham+RvD1高剂量组 6 25.23±8.90 136.63±16.74 37.75±10.19 IR+PBS组 6 101.28±12.311) 697.88±152.131) 287.65±62.081) IR+RvD1低剂量组 6 86.83±18.39 580.65±94.91 318.67±76.37 IR+RvD1中剂量组 6 42.47±12.942) 399.13±75.172) 83.55±12.342) IR+RvD1高剂量组 6 41.22±9.992) 400.15±121.562) 86.68±18.802) F值 42.276 36.249 56.206 P值 <0.001 <0.001 <0.001 注:与sham+PBS组比较,1)P<0.05;与IR+PBS组比较,2)P<0.05。 2.4 不同剂量RvD1对HO-1表达的影响
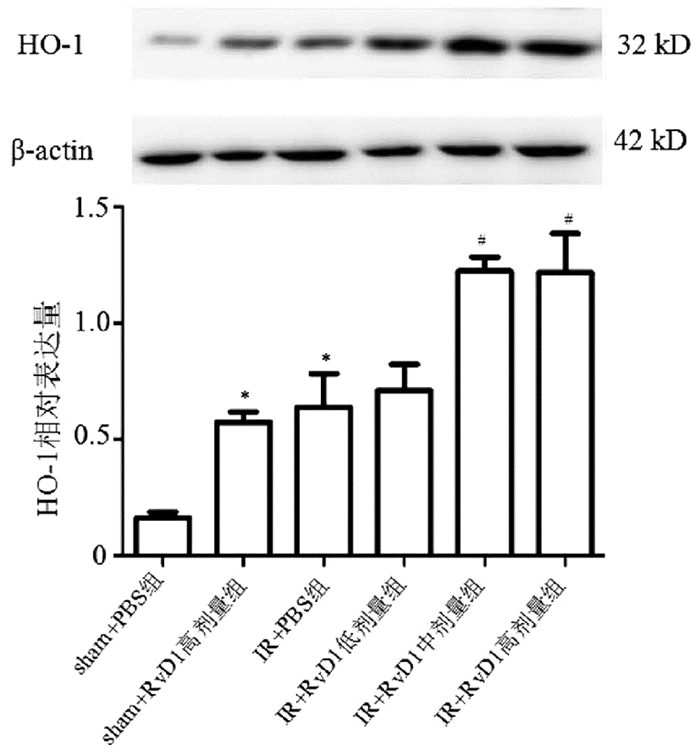
再灌注8 h后,与IR+PBS组相比,IR+RvD1中剂量和IR+RvD1高剂量组的HO-1表达均明显升高(P值均<0.05),但中、高剂量HO-1表达无显著差异(P>0.05)。与sham+PBS组相比,sham+RvD1高剂量组和IR+PBS组的HO-1表达亦明显增加(P值均<0.05)(图 2)。
3. 讨论
RvD是一种新型多不饱和脂肪酸衍生物,内源性RvD在炎症消退过程中自然产生,合成过程主要由多种脂氧合酶(lipoxygenase, LOX)催化。DHA先由15-LOX催化生成17S-羟过氧-DHA,然后在白细胞中被迅速转化为7S(8)-环氧化物中间体与4S(5)-环氧化物中间体,再经5-LOX脂氧化后水解形成RvD。RvD可与脂氧素A4受体或G蛋白偶联的GRP32受体结合,通过激活cAMP-PKA、PI3K-Akt等通路发挥多种生物学效应[10]。有研究[11]显示,在肝细胞的培养基内给予RvD1预处理,可以降低内质网应激导致的凋亡、胆固醇结合元件调节蛋白-1的表达及甘油三酯的沉积。因此,RvD1具有抗氧化、抗凋亡、抑制炎症反应等功能,并且因其副作用极少,非常适合应用于临床。
肝脏IR损伤是由于短暂缺血后血流再灌注带来的一系列氧化应激炎症反应,多见于消化道大出血、肝脏移植、肝脏部分切除及休克等临床状态,是导致患者死亡及移植手术失败的主要原因[12]。IR发生时出现的炎症反应以及炎症介质的级联放大反应、细胞凋亡、氧化应激均是IR发生发展的核心机制[13]。IR过程中可以诱导黏附分子表达,导致白细胞聚集、激活,释放各种炎症因子,包括TNFα、IL-6、IL-8等。炎症因子与相应受体结合后可引起细胞及组织的损伤。TNFα是17 kD的小分子蛋白质,由157个氨基酸组成的同型三聚体[14],一般可以由巨噬细胞、T淋巴细胞以及许多其他的细胞,如B淋巴细胞、中性粒细胞及上皮细胞分泌,在创伤、肝脏移植以及大量缺血时分泌明显增加,同时能够加强自身的止血功能[15]。在HCV及非酒精性脂肪性肝病导致的肝损伤中,Kupffer细胞、单核细胞、巨噬细胞分泌大量的TNFα、IL-6,加重肝细胞损伤[16]。故降低炎症因子水平[17]、抑制黏附分子表达[18]、缺血预处理[19]、抗氧化及抗凋亡等均能够对肝脏IR损伤起到保护作用。近些年,对于再灌注过程中各种炎症因子所发挥的作用,逐渐被越来越多的消化科及肝胆外科医生所关注[20]。
HO是血红素代谢过程中的限速酶,催化分解血红素生成一氧化碳、胆绿素和二价铁离子,其中HO-1与IR关系密切,主要分布于单核-吞噬细胞系统/巨噬细胞系统,在心、肺、肾、肝脏、脾脏、骨髓和网状内皮细胞等组织器官中表达较高[21]。HO-1蛋白质分子量约为32 kD,其编码基因Hmox-1位于染色体2q12,由5个外显子和4个内含子、1个近端增强子及2个远端增强子组成。生理状态下,HO-1表达量极低,而缺氧、氧化应激、氧化型低密度脂蛋白、内毒素、细胞因子等因素可使其表达明显升高。Cremers等[22]发现,缺血预处理可以上调HO-1表达。笔者前期研究[8]表明,HO-1激动剂预处理使大鼠肝脏中Bcl-2表达显著上调,抑制IR引起的肝细胞凋亡,缓解肝组织损伤;HO受抑制后肝组织损伤加重。HO-1缓解IR损伤的可能途径:(1)HO-1抗凋亡及细胞保护作用与其催化产物(一氧化碳、胆绿素和铁离子)有关。Li等[23]报道,一氧化碳激活可溶性鸟苷酸环化酶,使细胞内环磷酸鸟苷水平上升,进而发挥抗凋亡作用。一氧化碳还具有抗氧化、抗炎、改善微循环的作用[24]。胆绿素由于其生物化学特性而成为一种极强的抗氧化剂,可以有效清除ROS,从而抑制细胞凋亡[25]。根据其化学特性,铁离子可以具有氧化性,通过Fenton反应促进ROS产生。同时HO-1表达上调、铁离子增多时,又可诱导铁蛋白表达。而铁蛋白不仅能够抑制铁离子的氧化性,还可以发挥细胞保护作用[26]。(2)HO-1的细胞保护作用还与血红素的清除有关。细胞损伤后可产生游离血红素,后者促进ROS产生,损伤细胞膜、细胞骨架以及DNA等。而HO-1可以催化血红素分解代谢,同时反应产生的一氧化碳又可结合血红素,并抑制其氧化活性[27]。(3)HO-1抗氧化作用可以不依赖于其催化活性。Lin等[28]报道,HO-1可以进入细胞核,调节相关基因转录,进而发挥抗氧化作用。总之,HO-1具有抗氧化、抗凋亡、抗炎症、改善微循环等作用,可以缓解IR造成的组织损伤[29]。
笔者延用以往的肝脏IR动物模型,在相对成熟的实验动物模型体内验证RvD1对于肝脏IR的保护作用。本研究证实,一定剂量的RvD1能够降低再灌注肝脏的ALT和AST水平,发挥对肝脏IR损伤的保护作用。同时观察到,RvD1对炎症因子TNFα、IL-6和IL-8也有明显的下调作用,但继续增加RvD1的剂量时,该效应没有表现为持续增加。故RvD1对于肝脏IR的保护作用与其对炎症因子的抑制作用有关,并且具有一定程度的剂量依赖性。同时,本实验发现,RvD1对大鼠肝脏IR损伤的保护作用与HO-1表达的增加呈一致性表现,提示RvD1可能通过增加HO-1水平发挥对大鼠肝脏IR损伤的保护作用。前期试验及其他文献[8, 17]已经证实,HO-1对于肝脏IR损伤的保护作用可能与抑制炎症因子的表达和肝细胞凋亡有关。但是RvD1具体是通过哪种信号通路激活HO-1有待进一步研究。本研究为IR造成的肝损伤提供了一个新颖的治疗靶点,并且RvD1为不饱和脂肪酸衍生物,副作用较小,适宜推广至临床应用。
-
表 1 实验动物分组
组别 过程 缺血前1 h(腹腔注射) 缺血 缺血45 min后再灌注 再灌注8 h sham+PBS组 PBS 1 ml 开关腹 取标本 sham+RvD1高剂量组 RvD1 10 μg/kg 开关腹 取标本 IR+PBS组 PBS 1 ml 钳夹缺血 再灌注,关腹 取标本 IR+RvD1低剂量组 RvD1 2 μg/kg 钳夹缺血 再灌注,关腹 取标本 IR+RvD1中剂量组 RvD1 5 μg/kg 钳夹缺血 再灌注,关腹 取标本 IR+RvD1高剂量组 RvD1 10 μg/kg 钳夹缺血 再灌注,关腹 取标本 表 2 各组大鼠血浆转氨酶水平比较
组别 动物数(只) ALT(U/L) AST(U/L) sham+PBS组 6 56.88±11.65 58.45±12.11 sham+RvD1高剂量组 6 56.98±14.56 49.78±15.04 IR+PBS组 6 498.68±111.581) 606.35±150.061) IR+RvD1低剂量组 6 578.23±89.50 610.92±77.30 IR+RvD1中剂量组 6 373.13±105.592) 350.72±95.422) IR+RvD1高剂量组 6 348.17±86.522) 369.72±94.252) F值 43.926 47.459 P值 <0.001 <0.001 注:与sham+PBS组比较,1)P<0.05;与IR+PBS组比较,2)P<0.05。 表 3 各组大鼠血浆炎症因子TNFα、IL-6、IL-8水平比较
组别 动物数(只) IL-6(pg/ml) IL-8(pg/ml) TNFα(pg/ml) sham+PBS组 6 26.32±7.05 120.83±19.17 37.30±6.59 sham+RvD1高剂量组 6 25.23±8.90 136.63±16.74 37.75±10.19 IR+PBS组 6 101.28±12.311) 697.88±152.131) 287.65±62.081) IR+RvD1低剂量组 6 86.83±18.39 580.65±94.91 318.67±76.37 IR+RvD1中剂量组 6 42.47±12.942) 399.13±75.172) 83.55±12.342) IR+RvD1高剂量组 6 41.22±9.992) 400.15±121.562) 86.68±18.802) F值 42.276 36.249 56.206 P值 <0.001 <0.001 <0.001 注:与sham+PBS组比较,1)P<0.05;与IR+PBS组比较,2)P<0.05。 -
[1] JIMÉNEZ-CASTRO MB, CORNIDE-PETRONIO ME, GRACIA-SANCHO J, et al. Inflammasome-mediated inflammation in liver ischemia-reperfusion injury[J]. Cells, 2019, 8(10): 1131. DOI: 10.3390/cells8101131. [2] ZABALA V, BOYLAN JM, THEVENOT P, et al. Transcriptional changes during hepatic ischemia-reperfusion in the rat[J]. PLoS One, 2019, 14(12): e0227038. DOI: 10.1371/journal.pone.0227038. [3] WEYLANDT KH, CHIU CY, GOMOLKA B, et al. Omega-3 fatty acids and their lipid mediators: Towards an understanding of resolvin and protectin formation[J]. Prostaglandins Other Lipid Mediat, 2012, 97(3-4): 73-82. DOI: 10.1016/j.prostaglandins.2012.01.005. [4] GIACOBBE J, BENOITON B, ZUNSZAIN P, et al. The anti-inflammatory role of omega-3 polyunsaturated fatty acids metabolites in pre-clinical models of psychiatric, neurodegenerative, and neurological disorders[J]. Front Psychiatry, 2020, 11: 122. DOI: 10.3389/fpsyt.2020.00122. [5] LUO X, GU Y, TAO X, et al. Resolvin D5 inhibits neuropathic and inflammatory pain in male but not female mice: Distinct actions of D-series resolvins in chemotherapy-induced peripheral neuropathy[J]. Front Pharmacol, 2019, 10: 745. DOI: 10.3389/fphar.2019.00745. [6] SUN Z, WANG F, YANG Y, et al. Resolvin D1 attenuates ventilator-induced lung injury by reducing HMGB1 release in a HO-1-dependent pathway[J]. Int Immunopharmacol, 2019, 75: 105825. DOI: 10.1016/j.intimp.2019.105825. [7] KRASHIA P, CORDELLA A, NOBILI A, et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson's disease[J]. Nat Commun, 2019, 10(1): 3945. DOI: 10.1038/s41467-019-11928-w. [8] WANG YY, FENG ZJ, YUE YY, et al. Effect of heme oxygenase on apoptosis and apoptosis genes in hepatic ischemia reperfusion injury in rats[J]. Chin J Hepatol, 2007, 15(12): 922-925. DOI: 10.3760/j.issn:1007-3418.2007.12.011.王阳阳, 冯志杰, 岳媛媛, 等. 血红素氧合酶对大鼠肝脏缺血再灌注损伤细胞凋亡及相关基因的影响[J]. 中华肝脏病杂志, 2007, 15(12): 922-925. DOI: 10.3760/j.issn:1007-3418.2007. 12.011. [9] NAUTA RJ, TSIMOYIANNIS E, URIBE M, et al. Oxygen-derived free radicals in hepatic ischemia and reperfusion injury in the rat[J]. Surg Gynecol Obstet, 1990, 171(2): 120-125. http://europepmc.org/abstract/med/2382188 [10] LEE HJ, PARK MK, LEE EJ, et al. Resolvin D1 inhibits TGF-β1- induced epithelial mesenchymal transition of A549 lung cancer cells via lipoxin A4 receptor/formyl peptide receptor 2 and GPR32[J]. Int J Biochem Cell Biol, 2013, 45(12): 2801-2807. DOI: 10.1016/j.biocel.2013.09.018. [11] MUSSO G, GAMBINO R, CASSADER M, et al. Specialized proresolving mediators: Enhancing nonalcoholic steatohepatitis and fibrosis resolution[J]. Trends Pharmacol Sci, 2018, 39(4): 387-401. DOI: 10.1016/j.tips.2018.01.003. [12] ZHANG T, GU J, GUO J, et al. Renalase attenuates mouse fatty liver ischemia/reperfusion injury through mitigating oxidative stress and mitochondrial damage via activating SIRT1[J]. Oxid Med Cell Longev, 2019, 2019: 7534285. DOI: 10.1155/2019/7534285. [13] KONISHI T, LENTSCH AB. Hepatic ischemia/reperfusion: Mechanisms of tissue injury, repair, and regeneration[J]. Gene Expr, 2017, 17(4): 277-287. DOI: 10.3727/105221617X 15042750874156. [14] JOSEPHS SF, ICHIM TE, PRINCE SM, et al. Unleashing endogenous TNF-alpha as a cancer immunotherapeutic[J]. J Transl Med, 2018, 16(1): 242. DOI: 10.1186/s12967-018-1611-7. [15] KANY S, VOLLRATH JT, RELJA B. Cytokines in inflammatory disease[J]. Int J Mol Sci, 2019, 20(23): 6008. DOI: 10.3390/ijms20236008. [16] KHAN HA, AHMAD MZ, KHAN JA, et al. Crosstalk of liver immune cells and cell death mechanisms in different murine models of liver injury and its clinical relevance[J]. Hepatobiliary Pancreat Dis Int, 2017, 16(3): 245-256. DOI: 10.1016/s1499-3872(17)60014-6. [17] WANG YY, YU HL, LI TY, et al. Protective effect of HO-1 mediated mangiferin on liver ischemia/reperfusion injury in rats[J]. Chin Pharmacol Bull, 2015, 31(5): 736-737. DOI: 10.3969/j.issn.1001-1978.2015.05.028.王阳阳, 于惠玲, 李田阳, 等. HO-1介导芒果苷对大鼠肝脏缺血/再灌注损伤的保护作用[J]. 中国药理学通报, 2015, 31(5): 736-737. DOI: 10.3969/j.issn.1001-1978.2015.05.028. [18] WANG YY, MA YM, TIAN Y, et al. Effect of sEH inhibitor on hepatic I/R injury in rats and its mechanisms[J]. Chin Pharmacol Bull, 2013, 29(4): 590-591. DOI: 10.3969/j.issn.1001-1978.2013.04.033.王阳阳, 马幼敏, 田媛, 等. sEH抑制剂对大鼠肝缺血/再灌注损伤的影响及其机制[J]. 中国药理学通报, 2013, 29(4): 590-591. DOI: 10.3969/j.issn.1001-1978.2013.04.033. [19] YANG W, CHEN J, MENG Y, et al. Novel targets for treating ischemia-reperfusion injury in the liver[J]. Int J Mol Sci, 2018, 19(5): 1302. DOI: 10.3390/ijms19051302. [20] CORNIDE-PETRONIO ME, ÁLVAREZ-MERCADO AI, JIMÉNEZ-CASTRO MB, et al. Current knowledge about the effect of nutritional status, supplemented nutrition diet, and gut microbiota on hepatic ischemia-reperfusion and regeneration in liver surgery[J]. Nutrients, 2020, 12(2): 284. DOI: 10.3390/nu12020284. [21] ZHU LJ, YUAN SF, SHANGGUAN ZX, et al. miR-1304 inhibits non-small cell lung cancer cell invasion and prolifer-ation in vitro via targeting heme oxygenase-1[J]. Chin J Clin Pharmacol Ther, 2019, 24(4): 383-390. DOI: 10.12092/j.issn.1009-2501.2019.04.004.朱林佳, 原少斐, 上官宗校, 等. microRNA-1304通过靶向血红素氧合酶-1对人肺癌细胞的抑制作用[J]. 中国临床药理学与治疗学, 2019, 24(4): 383-390. DOI: 10.12092/j.issn.1009-2501.2019.04.004. [22] CREMERS NA, WEVER KE, WONG RJ, et al. Effects of remote ischemic preconditioning on heme oxygenase-1 expression and cutaneous wound repair[J]. Int J Mol Sci, 2017, 18(2): 438. DOI: 10.3390/ijms18020438. [23] LI L, LI CM, WU J, et al. Heat shock protein 32/heme oxygenase-1 protects mouse Sertoli cells from hyperthermia-induced apoptosis by CO activation of sGC signalling pathways[J]. Cell Biol Int, 2014, 38(1): 64-71. DOI: 10.1002/cbin.10177. [24] CHEN XP, FENG ZJ. System of heme oxygenase-carbon monoxide and liver oxidative stress[J]. J Clin Hepatol, 2005, 21(5): 309-311. http://lcgdbzz.org/article/id/LCGD200505029陈湘萍, 冯志杰. 血红素氧合酶-一氧化碳系统与肝脏的氧化应激[J]. 临床肝胆病杂志, 2005, 21(5): 309-311. http://lcgdbzz.org/article/id/LCGD200505029 [25] WEGIEL B, NEMETH Z, CORREA-COSTA M, et al. Heme oxygenase-1: A metabolic nike[J]. Antioxid Redox Signal, 2014, 20(11): 1709-1722. DOI: 10.1089/ars.2013.5667. [26] JIN HH, LI ZP. Correlational research of serum ferritin and hyaluronic acid in patients with cirrhosis[J]. J Clin Hepatol, 2012, 28(3): 216-218. http://lcgdbzz.org/article/id/LCGD201203017金宏慧, 李仲平. 血清铁蛋白与透明质酸和肝硬化的相关性[J]. 临床肝胆病杂志, 2012, 28(3): 216-218. http://lcgdbzz.org/article/id/LCGD201203017 [27] BARBAGALLO I, NICOLOSI A, CALABRESE G, et al. The role of the heme oxygenase system in the metabolic syndrome[J]. Curr Pharm Des, 2014, 20(31): 4970-4974. DOI: 10.2174/1381612819666131206103824. [28] LIN Q, WEIS S, YANG G, et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress[J]. J Biol Chem, 2007, 282(28): 20621-20633. DOI: 10.1074/jbc.M607954200. [29] MAHMOUD AM, HUSSEIN OE, HOZAYEN WG, et al. Ferulic acid prevents oxidative stress, inflammation, and liver injury via upregulation of Nrf2/HO-1 signaling in methotrexate-induced rats[J]. Environ Sci Pollut Res Int, 2020, 27(8): 7910-7921. DOI: 10.1007/s11356-019-07532-6. 期刊类型引用(3)
1. 罗嘉琪,王莉荔,陈阜东,张爱舷,张晗,张晓萌,陈力. 4种急性肝缺血/再灌注损伤大鼠模型的建立及适用性比较. 中华危重病急救医学. 2023(06): 604-609 .  百度学术
百度学术2. 王燕平,曹艳敏,王新蕾. 消退素D1对原位肝癌的抗癌作用及其对Th1/Th2平衡的影响. 免疫学杂志. 2023(09): 754-760 .  百度学术
百度学术3. 王阳阳,赵娜,彭雪莹,李慧,王景艳. 消退素D1对大鼠肝缺血再灌注损伤的保护作用. 医学研究与教育. 2021(06): 1-7 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 2211 KB)
PDF下载 ( 2211 KB)

 下载:
下载:



 下载:
下载:

 百度学术
百度学术