实时多影像融合介入导航系统引导下纳米刀消融治疗局部进展期胰腺癌的效果与安全性分析
DOI: 10.3969/j.issn.1001-5256.2021.06.033
Effectiveness and safety of nanoknife ablation guided by real-time virtual sonography in treatment of locally advanced pancreatic cancer
-
摘要:
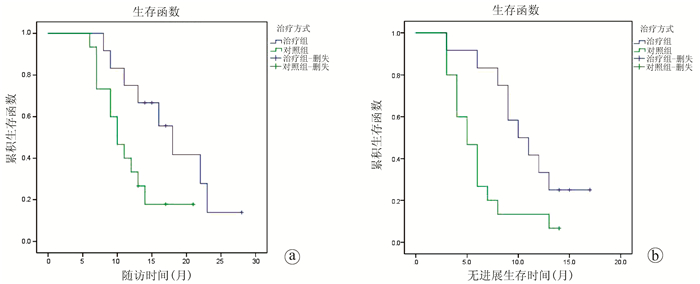
目的 探讨实时多影像融合介入导航系统引导下纳米刀消融治疗局部进展期胰腺癌(LAPC)的效果与安全性。 方法 回顾性收集2018年4月—2019年10月于郑州大学第五附属医院就诊的LAPC患者的临床资料,按治疗方式分为不可逆电穿孔(IRE)联合化疗组(联合组)12例,单纯化疗组(对照组)15例,化疗方案均为吉西他滨+替吉奥。观察联合组围手术期内的不良反应及并发症,比较联合组术前与术后不同时间点心肌酶、血淀粉酶指标变化,比较2组治疗前后CA19-9水平变化,比较2组治疗后3个月的疾病缓解率(RR)、疾病控制率(DCR)及随访期内的生存状况。非正态分布的计量资料组间比较采用Mann-Whitney U检验,组内比较采用Wilcoxon秩和检验;计数资料2组间比较采用Fisher精确检验;Kaplan-Meier法分析患者随访期内的生存状态。 结果 联合组围手术期内出现不良反应及轻度并发症12例次(Clavien-DindoⅠ级9例次、Ⅱ级3例次)。联合组所有患者术后心肌酶指标一过性升高,7 d内恢复正常,术前与术后7 d肌酸激酶、乳酸脱氢酶水平无统计学差异(P值均>0.05);术后1 d,9例患者血淀粉酶水平明显升高,至术后7 d明显下降,术后14 d基本恢复正常,术前与术后7、14 d、1个月血淀粉酶水平无统计学差异(P值均>0.05)。2组患者治疗前CA19-9水平均高于正常值,联合组治疗后CA19-9水平逐步下降,比较治疗前与治疗后1、2、3个月CA19-9水平,差异有统计学意义(P值均<0.05);对照组治疗后CA19-9水平呈短暂下降随后上升,但治疗前与治疗后1、2、3个月CA19-9水平差异无统计学意义(P值均>0.05)。治疗后3个月,联合组RR、DCR均高于对照组(75.0% vs 26.7%、91.6% vs 53.3%,P值分别为0.021、0.043)。在中位随访期(13个月)内,联合组中位无进展生存期、中位总生存期均高于对照组,差异有统计学意义(10个月vs 5个月、18个月vs 10个月,P值分别为0.014、0.034)。 结论 实时多影像融合系统引导下经皮穿刺纳米刀消融治疗LAPC安全性高,疗效肯定,可作为不耐受开腹或拒绝开腹消融患者的一种全新治疗选择。 Abstract:Objective To investigate the effectiveness and safety of nanoknife ablation guided by real-time virtual sonography (RVS) in the treatment of locally advanced pancreatic cancer (LAPC). Methods A retrospective analysis was performed for the clinical data of 27 patients with LAPC who attended The Fifth Affiliated Hospital of Zhengzhou University from April 2018 to October 2019, and according to the treatment method, the patients were divided into combination group (12 patients treated with IRE combined with chemotherapy) and control group (15 patients treated with chemotherapy alone). The chemotherapy regimen was gemcitabine combined with tegafur, gimeracil and oteracil potassium for both groups. Adverse reactions and complications were observed for the combination group during the perioperative period, and the two groups were compared in terms of the changes in myocardial enzymes, blood amylase, and carbohydrate antigen 19-9 (CA19-9) before treatment and at different time points after treatment, as well as remission rate (RR) and disease control rate (DCR) at 3 months after treatment and survival status during follow-up. The Mann-Whitney U test was used for comparison of non-normally distributed continuous data between groups, and the Wilcoxon test was used for comparison within each group; the Fisher's exact test was used for comparison of categorical data between groups; the Kaplan-Meier method was used to analyze the survival status during follow-up. Results In the combination group, there were 12 cases of adverse reactions and mild complications during the perioperative period, i.e., 9 Clavien-Dindo grade Ⅰ cases and 3 grade Ⅱ cases. All patients in the combination group experienced a transient increase in myocardial enzymes, which returned to normal within 7 days, and there were no significant changes in creatine kinase and lactate dehydrogenase on day 7 after treatment (P > 0.05); 9 patients had a significant increase in blood amylase on day 1 after surgery, which significantly decreased on day 7 after surgery and basically returned to normal on day 14 after surgery, and there was no significant change in blood amylase on days 7、14, and 1 month after surgery (P > 0.05). Before treatment, the level of CA19-9 was higher than the normal value in both groups, and the combination group had a significant reduction in CA19-9 at 1, 2, and 3 months after treatment (all P < 0.05); in the control group, the level of CA19-9 firstly decreased for a short time and then increased, while there was no significant change in CA19-9 at 1, 2, and 3 months after treatment (all P > 0.05). At 3 months after treatment, the combination group had significantly higher RR and DCR than the control group (RR: 75.0% vs 26.7%, P=0.021; DCR: 91.6% vs 53.3%, P=0.043). During the median follow-up time of 13 months, compared with the control group, the combination group had significantly higher median progression-free survival time (10 months vs 5 months, P=0.014) and median overall survival time (18 months vs 10 months, P=0.034). Conclusion RVS-guided percutaneous nanoknife ablation has marked clinical effect and high safety in the treatment of LAPC and can be used as a new treatment option for patients who refuse or cannot tolerate laparotomy for ablation therapy. -
Key words:
- Pancreatic Neoplasms /
- Radiology, Interventional /
- Catheter Ablation
-
表 1 mRECIST评价标准
疗效评估 定义 完全缓解(CR) 病灶动脉期增强显影均消失 部分缓解(PR) 靶病灶动脉期增强显影的直径至少减少30% 疾病稳定(SD) 既达不到部分缓解标准,又达不到进展标准者 疾病进展(PD) 靶病灶动脉期增强显影直径至少增加20%或出现新的病灶 疾病控制率(DCR) (CR+PR+SD)/总例数×100% 疾病缓解率(RR) (CR+PR)/总例数×100% 表 2 2组患者一般资料比较
指标 联合组(n=12) 对照组(n=15) 统计值 P值 性别(例) 0.3431) 男/女 5/7 9/6 年龄(岁) 62.0(51.5~67.5) 59.0(54.0~66.0) Z=-0.465 0.642 肿瘤部位(例) 0.7061) 胰头/胰颈/胰体尾 3/5/4 4/7/4 肿瘤最大直径(cm) 3.80(3.50~4.83) 3.70(3.50~4.40) Z=-0.294 0.769 肿瘤分期(例) 0.7221) Ⅱ期/Ⅲ期 4/6 8/9 注:1)Fisher精确检验。 表 3 联合组术前与术后不同时间点心肌酶指标、血淀粉酶水平变化比较
项目 例数 术前1 d 术后1 d 术后3 d 术后7 d 肌酸激酶(U/L) 12 61.5(39.8~74.0) 156.0(128.3~186.0)1) 73.0(64.3~89.5)1) 60.0(39.3~76.3) 乳酸脱氢酶(U/L) 12 150.0(128.3~172.5) 326.0(293.8~373.3)1) 167.5(142.0~179.5)1) 143.0(115.8~170.8) 血淀粉酶(U/L) 9 68.0(38.5~75.0) 216.0(161.5~361.5)1) 103.0(83.5~164.5)1) 65.0(33.5~84.0) 注:与术前1 d比较, 1)P<0.05。 表 4 2组治疗前后不同时间点CA19-9水平变化比较
组别 例数 术前 术后7 d 术后14 d 术后1个月 术后2个月 术后3个月 联合组(U/ml) 12 564.0(248.2~696.0) 536.8(208.7~1000.0) 301.1(178.1~417.9) 284.4(98.8~313.0)1) 122.8(93.4~221.9)1) 94.7(80.3~209.9)1) 对照组(U/ml) 15 619.9(381.8~857.4) 476.5(321.7~672.5) 358.4(312.4~674.2) 299.0(216.5~571.1) 599.3(286.5~825.1) 581.0(373.2~976.5) 注:与术前比较,1)P<0.05。 表 5 2组患者治疗后3个月mRECIST标准评价肿瘤治疗效果
组别 例数 CR PR SD PD RR(%) DCR(%) 联合组 12 4 5 2 1 75.0 91.6 对照组 15 0 4 4 7 26.7 53.3 -
[1] Pancreatic Cancer Committee of Chinese Anti-Cancer Association. Comprehensive guidelines for the diagnosis and treatment of pancreatic cancer (2018 version)[J]. J Clin Hepatol, 2018, 34(10): 2109-2120. DOI: 10.3969/j.issn.1001-5256.2018.10.011.中国抗癌协会胰腺癌专业委员会. 胰腺癌综合治疗指南(2018版)[J]. 临床肝胆病杂志, 2018, 34(10): 2109-2120. DOI: 10.3969/j.issn.1001-5256.2018.10.011. [2] HE C, WANG J, ZHANG Y, et al. Comparison of combination therapies in the management of locally advanced pancreatic cancer: Induction chemotherapy followed by irreversible electroporation vs radiofrequency ablation[J]. Cancer Med, 2020, 9(13): 4699-4710. DOI: 10.1002/cam4.3119. [3] PAIELLA S, de PASTENA M, D'ONOFRIO M, et al. Palliative therapy in pancreatic cancer-interventional treatment with radiofrequency ablation/irreversible electroporation[J]. Transl Gastroenterol Hepatol, 2018, 3: 80. DOI: 10.21037/tgh.2018.10.05. [4] HAMMEL P, HUGUET F, van LAETHEM JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: The LAP07 randomized clinical trial[J]. JAMA, 2016, 315(17): 1844-1853. DOI: 10.1001/jama.2016.4324. [5] VROOMEN L, PETRE EN, CORNELIS FH, et al. Irreversible electroporation and thermal ablation of tumors in the liver, lung, kidney and bone: What are the differences?[J]. Diagn Interv Imaging, 2017, 98(9): 609-617. DOI: 10.1016/j.diii.2017.07.007. [6] VOGEL JA, van VELDHUISEN E, AGNASS P, et al. Time-dependent impact of irreversible electroporation on pancreas, liver, blood vessels and nerves: A systematic review of experimental studies[J]. PLoS One, 2016, 11(11): e0166987. DOI: 10.1371/journal.pone.0166987. [7] van ROESSEL S, KASUMOVA GG, VERHEIJ J, et al. International validation of the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system in patients with resected pancreatic cancer[J]. JAMA Surg, 2018, 153(12): e183617. DOI: 10.1001/jamasurg.2018.3617. [8] LENCIONI R, LLOVET JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma[J]. Semin Liver Dis, 2010, 30(1): 52-60. DOI: 10.1055/s-0030-1247132. [9] RUARUS A, VROOMEN L, PUIJK R, et al. Locally advanced pancreatic cancer: A review of local ablative therapies[J]. Cancers (Basel), 2018, 10(1): 16. DOI: 10.3390/cancers10010016. [10] Chinese Society of Interventional and Minimally Invasive Therapy, China Medicine Education Association. Expert consensus on image-guided irreversible electroporation ablation for pancreatic cancer (2018 version)[J]. J Clin Hepatol, 2019, 35(2): 299-302. DOI: 10.3969/j.issn.1001-5256.2019.02.011中国医药教育协会介入微创治疗专业委员会. 影像学引导胰腺癌不可逆电穿孔消融治疗专家共识2018版[J]. 临床肝胆病杂志, 2019, 35(2): 299-302. DOI: 10.3969/j.issn.1001-5256.2019.02.011. [11] AL EFISHAT M, WOLFGANG CL, WEISS MJ. Stage Ⅲ pancreatic cancer and the role of irreversible electroporation[J]. BMJ, 2015, 350: h521. DOI: 10.1136/bmj.h521. [12] HOU JY, LIU RB, LIU Y, et al. The value of radiofrequency ablation in the special sites of the liver cancer by combining ultrasound guided with CT monitoring[J]. J Clin Radiol, 2012, 31(7): 1014-1017. https://www.cnki.com.cn/Article/CJFDTOTAL-LCFS201207029.htm侯继野, 刘瑞宝, 刘岩, 等. 超声引导联合CT监视在特殊部位肝癌射频治疗中的应用价值[J]. 临床放射学杂志, 2012, 31(7): 1014-1017. https://www.cnki.com.cn/Article/CJFDTOTAL-LCFS201207029.htm [13] BELFIORE MP, RONZA FM, ROMANO F, et al. Percutaneous CT-guided irreversible electroporation followed by chemotherapy as a novel neoadjuvant protocol in locally advanced pancreatic cancer: Our preliminary experience[J]. Int J Surg, 2015, 21(Suppl 1): s34-s39. DOI: 10.1016/j.ijsu.2015.06.049. [14] ZHANG Y, SHI J, ZENG J, et al. Percutaneous irreversible electroporation for ablation of locally advanced pancreatic cancer: Experience from a Chinese institution[J]. Pancreas, 2017, 46(2): e12-12e14. DOI: 10.1097/MPA.0000000000000703. [15] SCHEFFER HJ, VROOMEN LG, de JONG MC, et al. Ablation of locally advanced pancreatic cancer with percutaneous irreversible electroporation: Results of the Phase Ⅰ/Ⅱ PANFIRE Study[J]. Radiology, 2017, 282(2): 585-597. DOI: 10.1148/radiol.2016152835. [16] TONG H, LI XY, CHEN YJ, et al. Complications of nano-knife ablation on locally advanced pancreatic cancer[J]. Chin J Hepatobiliary Surg, 2020, 26(4): 270-271. DOI: 10.3760/cma.j.cn113884-20190923-00312.仝昊, 李晓勇, 陈艳军, 等. 局部进展期胰腺癌纳米刀消融术后并发症分析[J]. 中华肝胆外科杂志, 2020, 26(4) : 270-271. DOI: 10.3760/cma.j.cn113884-20190923-00312. [17] MORIS D, MACHAIRAS N, TSILIMIGRAS DI, et al. Systematic review of surgical and percutaneous irreversible electroporation in the treatment of locally advanced pancreatic cancer[J]. Ann Surg Oncol, 2019, 26(6): 1657-1668. DOI: 10.1245/s10434-019-07261-7. [18] MARTIN RC 2nd, KWON D, CHALIKONDA S, et al. Treatment of 200 locally advanced (stage Ⅲ) pancreatic adenocarcinoma patients with irreversible electroporation: Safety and efficacy[J]. Ann Surg, 2015, 262(3): 486-494; discussion 492-494. DOI: 10.1097/SLA.0000000000001441. [19] YANG PC, HUANG KW, PUA U, et al. Prognostic factor analysis of irreversible electroporation for locally advanced pancreatic cancer-A multi-institutional clinical study in Asia[J]. Eur J Surg Oncol, 2020, 46(5): 811-817. DOI: 10.1016/j.ejso.2019.12.006. -



 PDF下载 ( 2639 KB)
PDF下载 ( 2639 KB)

 下载:
下载: