合并与未合并糖尿病的胰腺癌患者血管生成素样蛋白2在血清中的表达及其与预后的关系
DOI: 10.3969/j.issn.1001-5256.2021.06.034
Serum expression of angiopoietin-like protein 2 in pancreatic cancer patients with or without diabetes and its association with prognosis
-
摘要:
目的 探讨血管生成素样蛋白2(ANGPTL2)在合并与未合并糖尿病的胰腺癌患者中的表达水平及其作为胰腺癌患者预后指标的价值。 方法 收集2015年1月—2018年1月于广西医科大学第一附属医院、广西医科大学附属肿瘤医院、广西医科大学附属武鸣医院治疗的胰腺癌患者的血清标本125例,其中单纯胰腺癌组64例、合并糖尿病胰腺癌组61例,另选取66例健康体检者作为对照组。采用ELISA法检测各组血清ANGPTL2水平,分析ANGPTL2的表达水平与临床指标、生存预后的关系。符合正态分布的计量资料3组间比较采用单因素方差分析,进一步两两比较采用Bonferroni法;偏态分布者的计量资料3组间比较采用独立样本Kruskal-Wallis H秩和检验,进一步两两比较采用单因素ANOVA法。计数资料组间比较采用χ2检验。相关性分析采用Spearman相关分析。Kaplan-Meier法绘制生存曲线,log-rank检验比较生存率。采用Cox风险模型行单/多因素分析,确定影响胰腺癌预后的独立危险因素。 结果 合并糖尿病胰腺癌组患者血清ANGPTL2[7.79(7.12~8.17) ng/ml]明显高于单纯胰腺癌组[5.74(5.08~6.40) ng/ml]和健康对照组[3.72(3.25~4.16) ng/ml](P值均<0.001)。血清ANGPTL2水平与CA19-9、CEA均呈正相关(r值分别为0.560、0.731,P值均<0.001)。单因素分析显示,肿瘤大小、远处器官转移、分化程度、CEA、ANGPTL2、HbA1c与胰腺癌患者远期生存密切相关(P值均<0.05);多因素分析显示,肿瘤大小(HR=2.657,P=0.005)、远处器官转移(HR=5.000,P=0.014)、分化程度(HR=2.466, P=0.004)、CEA(HR=1.110,P<0.001)、ANGPTL2(HR=1.901,P=0.001)均为影响胰腺癌患者预后的独立危险因素。在所有胰腺癌患者中,ANGPTL2高表达组的2年生存率明显低于ANGPTL2低表达组(8.51% vs 25.81%,χ2=5.651,P=0.017)。在合并糖尿病的胰腺癌患者中ANGPTL2高表达组的2年生存率也明显低于ANGPTL2低表达组(2.20% vs 32.70%, χ2=24.895,P<0.001)。 结论 ANGPTL2可能是评价胰腺癌患者,特别是合并糖尿病的胰腺癌患者预后的有效指标。 Abstract:Objective To investigate the expression level of angiopoietin-like protein 2 (ANGPTL2) in pancreatic cancer patients with or without diabetes and the clinical value of ANGPTL2 as a prognostic marker in patients with pancreatic cancer. Methods Serum samples were collected from 125 pancreatic cancer patients who were treated in The First Affiliated Hospital of Guangxi Medical University, Guangxi Medical University Cancer Hospital, and Wuming Hospital of Guangxi Medical University from January 2015 to January 2018, among whom 64 had pancreatic cancer alone and 61 had pancreatic cancer and diabetes, and 66 individuals who underwent physical examination were enrolled as control group. ELISA was used to measure the serum level of ANGPTL2, and the association of the expression level of ANGPTL2 with clinical indices, survival, and prognosis was analyzed. A one-way analysis of variance was used for comparison of normally distributed continuous data between three groups, and the Bonferroni test was used for comparison between two groups. The independent-samples Kruskal-Wallis H test was used for comparison of continuous data with skewed distribution between three groups and the one-way ANOVA analysis was used for comparison between two groups. The chi-square test was used for comparison of categorical data between groups. Spearman correlation analysis was also performed to investigate correlation. The Kaplan-Meier method was used to plot survival curves, and the log-rank test was used for comparison of survival rate. The Cox risk model was used to perform univariate and multivariate analyses to determine independent risk factors for the prognosis of pancreatic cancer. Results The pancreatic cancer+diabetes group had a significantly higher serum concentration of ANGPTL2 than the pancreatic cancer group and the control group [7.79 (7.12-8.17) ng/ml vs 5.74 (5.08-6.40) ng/ml and 3.72 (3.25-4.16) ng/ml, χ2=126.367, P < 0.001]. Serum ANGPTL2 concentration was positively correlated with carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA) (r=0.560 and 0.731, both P < 0.001). The univariate analysis showed that tumor size, distant organ metastasis, degree of tumor differentiation, CEA, ANGPTL2, and HbA1c were closely associated with the long-term survival of pancreatic cancer patients, and the multivariate analysis showed that tumor size (HR=2.657, P=0.005), distant organ metastasis (HR=5.000, P=0.014), degree of tumor differentiation (HR=2.466, P=0.004), CEA(HR=1.110, P < 0.001) and ANGPTL2(HR=1.901, P=0.001) were independent risk factors for the prognosis of pancreatic cancer patients. For all pancreatic cancer patients, the high ANGPTL2 expression group had a significantly lower 2-year survival rate than the low ANGPTL2 expression group (8.51% vs 25.81%, χ2=5.651, P=0.017). For the pancreatic cancer patients with diabetes, the high ANGPTL2 expression group had a significantly lower 2-year survival rate than the low ANGPTL2 expression group (2.20% vs 32.70%, χ2=24.895, P < 0.001). Conclusion ANGPTL2 can be used as an effective clinical index to evaluate the prognosis of pancreatic cancer patients, especially those with diabetes. -
Key words:
- Pancreatic Neoplasms /
- Diabetes Mellitus /
- Angiopoietins /
- Prognosis
-
胰腺癌是预后最差的恶性肿瘤之一,5年生存率<5%,且近年来患者数量持续增加。尽管医疗技术已取得重大进步,但胰腺癌存活期短的情况并未发生改变,这与胰腺癌难以早期诊断和缺乏有效的治疗药物有关。此外,大量研究[1-2]显示,糖尿病与胰腺癌之间存在复杂的关系,二者互为因果:一方面,多达45%的胰腺癌病例表现为新发糖尿病,即胰腺癌是引发糖尿病的原因之一;另一方面,≥5年的长期2型糖尿病者发生胰腺癌的风险增加50%[3],即长期存在的2型糖尿病是胰腺癌的危险因素。血管生成素样蛋白2(angiopoietin-like protein 2,ANGPTL2)是血管生成素蛋白家族的成员,其本质是一种脂肪来源的分泌糖蛋白[4]。在缺氧和内质网应激时,脂肪组织分泌的ANGPTL2增加。ANGPTL2由493个氨基酸组成,其结构包括N端的卷曲-卷曲结构域和C端的纤维蛋白原样结构域[5]。由于其在类纤维蛋白区域缺少一个半胱氨酸基序,无法与络氨酸受体Tie1、2相结合,这一结构特点使其功能更为广泛。据报道[6-8],ANGPTL2在糖尿病、代谢综合征、炎症性疾病和肿瘤性疾病中发挥多种重要作用。因此,笔者推测ANGPTL2水平变化可能引发糖尿病和肿瘤;若糖尿病与肿瘤叠加发生,则ANGPTL2水平变化可能更为显著。本研究旨在从临床角度探讨ANGPTL2在合并与未合并糖尿病的胰腺癌患者中的表达水平及其对预后的预测价值。
1. 资料与方法
1.1 研究对象
收集2015年1月—2018年1月于广西医科大学第一附属医院、广西医科大学附属肿瘤医院、广西医科大学附属武鸣医院就诊的胰腺癌患者的血清标本及临床资料。标本为患者首次就诊时检测CEA、CA19-9剩余血清样本,冻存于-20 ℃冰箱,以备ANGPTL2检测。纳入标准:(1)临床病理资料及随访资料完整,签署知情同意书,胰腺穿刺活组织检查或术后病理确诊为胰腺癌;(2)无严重的心、肝、肾功能不全。排除标准:(1)接受过抗癌治疗的患者,包括手术、放化疗、生物制剂、基因治疗和免疫治疗等;(2)合并其他恶性肿瘤患者。根据是否合并糖尿病将患者分为单纯胰腺癌组和合并糖尿病胰腺癌组,另选同期健康体检者作为正常对照组。
1.2 资料获取
通过医院病案信息系统进行检索,收集患者一般资料、入院时常规及肿瘤相关检验、检查数据。自患者出院起,每3个月电话随访以及信访,如果出现2年或以上无法联系者,则视为失访。病例随访时间为3年,随访内容包括出院后生活情况、是否死亡、死亡时间及死亡原因。如果在随访期间患者死于胰腺癌则患者资料为完全数据,如果至随访截止日期患者生存,则按截尾数据处理。
1.3 检测方法
ELISA测定血清ANGPTL2水平(USCN生命科学股份有限公司,中国武汉)。来自每个受试者的血清和标准蛋白ANGPTL2被缓冲液稀释,并加入预包被了ANGPTL2抗体的96孔板上。于37 ℃孵育2 h时后,将每孔中的液体移除,并加入检测试剂中的工作液A,于37 ℃孵育1 h。之后,加入检测试剂中的工作液B,于37 ℃孵育30 min。在每步操作之间,96孔板应洗5次,每次10 min。然后,在各孔中加入底物溶液、终止液,于37 ℃避光孵育15 min。最后,在450 nm处测定各孔板的吸光度。通过稀释标准化的重组ANGPTL2蛋白绘制标准曲线。根据标准曲线和样品3次测量的平均光密度,分别计算每孔的血清ANGPTL2浓度。
1.4 伦理学审查
本研究方案经由广西医科大学附属武鸣医院伦理委员会审批,批号:伦审WM-2019(100),研究对象均签署知情同意书。
1.5 统计学方法
采用SPSS 20.0软件进行统计学分析。符合正态分布的计量资料以x±s表示,3组间比较用单因素方差分析,进一步两两比较采用Bonferroni法;偏态分布的计量资料以M(P25~P75)表示,3组间比较采用独立样本Kruskal-Wallis H秩和检验,进一步两两比较采用单因素ANOVA法。计数资料组间比较采用χ2检验。相关性分析采用Spearman相关分析。Kaplan-Meier法绘制生存曲线,log-rank检验生存率。采用Cox风险模型行单/多因素分析,确定影响胰腺癌预后的独立危险因素。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
共纳入125例胰腺癌患者,分为单纯胰腺癌组64例,其中男35例,女29例,平均年龄(62.91±8.78)岁;合并糖尿病胰腺癌组61例,其中男34例,女27例,平均年龄(61.00±8.23)岁;另选正常对照组66例,其中男36例,女30例,平均年龄(63.08±8.25)岁。3组性别、年龄比较差异均无统计学意义(P值均>0.05)。
BMI、糖化血红蛋白(HbA1c)在3组间比较差异均有统计学意义(P值均<0.05)。合并糖尿病胰腺癌组的C反应蛋白(CRP)、甘油三酯(TG)水平均高于健康对照组,差异均有统计学意义(P值均<0.05),但与单纯胰腺癌组相比差异均无统计学意义(P值均>0.05)。合并糖尿病胰腺癌组的总胆固醇(TC)水平低于单纯胰腺癌组和健康对照组,差异均有统计学意义(P值均<0.05)(表 1)。
表 1 3组一般资料比较项目 单纯胰腺癌组(n=64) 合并糖尿病胰腺癌组(n=61) 健康对照组(n=66) 统计值 P值 年龄(岁) 63.08±8.25 61.00±8.23 62.91±8.78 F=1.166 0.314 性别(男/女) 35/29 34/27 36/30 χ2=0.021 0.989 BMI(kg/m2) 24.29±1.92 23.40±2.05 25.16±2.00 F=12.371 <0.001 HbA1c(%) 5.50±0.29 8.28±0.59 5.27±0.16 F=119.6 <0.001 CRP(mg/dl) 1.61±0.68 1.85±0.621) 0.25±0.12 F=170.022 <0.001 TC(mg/dl) 178.17±22.61 167.78±22.661)2) 186.00±22.34 F=10.394 <0.001 TG(mg/dl) 119.59±28.62 122.67±26.431) 97.59±25.55 F=16.696 <0.001 注:1)与健康对照组比较,P<0.05;2)与单纯胰腺癌组比较,P<0.05。 2.2 各组血清肿瘤相关指标水平比较
CEA、CA19-9、ANGPTL2水平由高至低为:合并糖尿病胰腺癌组、单纯胰腺癌组、健康对照组,差异均有统计学意义(P值均<0.001)(表 2)。
表 2 3组人群血清CEA、CA19-9及ANGPTL2水平比较组别 例数 CEA(ng/ml) CA19-9(U/ml) ANGPTL2(ng/ml) 单纯胰腺癌组 64 14.71(9.07~24.07) 83.11(40.85~109.77) 5.74(5.08~6.40) 合并糖尿病胰腺癌组 61 30.43(18.99~37.12) 130.45(98.62~214.01) 7.79(7.12~8.17) 健康对照组 66 2.01(1.42~2.93) 13.08(9.03~19.60) 3.72(3.25~4.16) χ2值 135.7011) 114.5181) 126.3671) P值 <0.001 <0.001 <0.001 注: 组间两两比较,1)P值均<0.05。 2.3 胰腺癌患者血清ANGPTL2水平与CA19-9、CEA的相关性
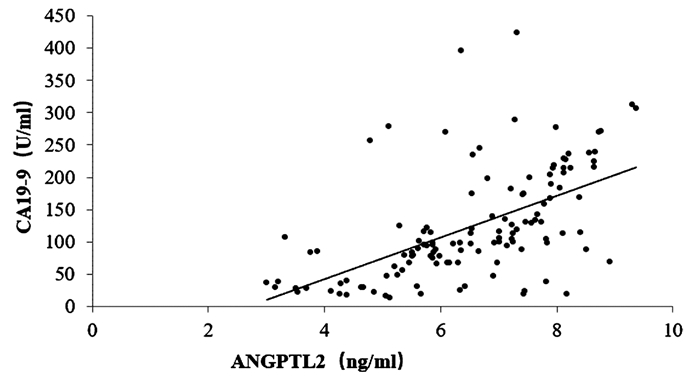
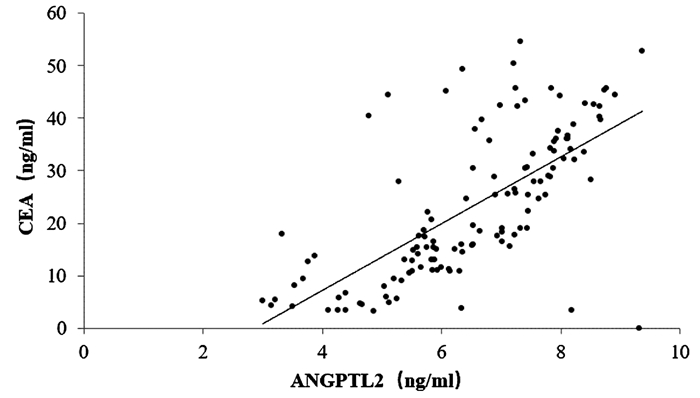
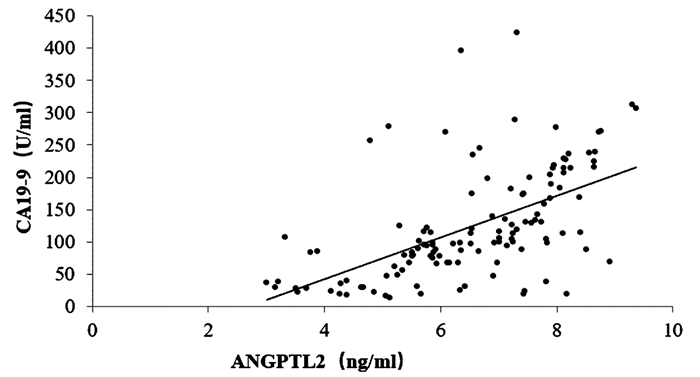
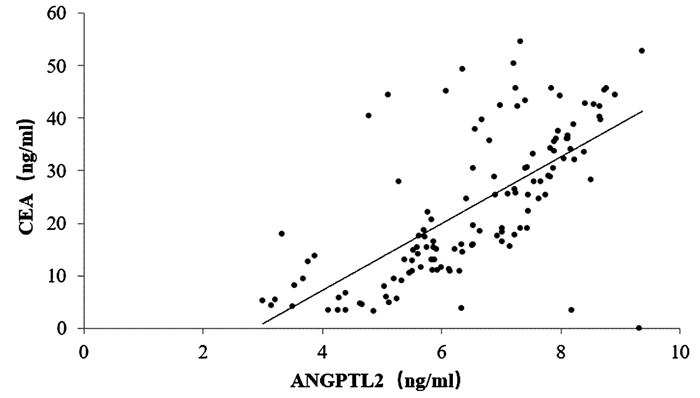
125例胰腺癌患者的血清ANGPTL2为6.56(5.58~7.89)ng/ml,CA19-9为118.43(79.76~165.32)U/ml,CEA为24.93(12.54~34.67)ng/ml。相关性分析结果显示,胰腺癌患者血清ANGPTL2与CA19-9、CEA均呈正相关(r值分别为0.560、0.731,P值均<0.001)(图 1、2)。
2.4 影响胰腺癌患者远期生存的单因素和多因素分析
单因素分析显示,肿瘤大小、远处器官转移、分化程度、CEA、ANGPTL2、HbA1c对胰腺癌患者远期生存有影响(P值均<0.05)(表 3)。多因素分析显示,肿瘤大小、远处器官转移、分化程度、CEA和ANGPTL2是影响胰腺癌患者预后的独立危险因素(P值均<0.05)(表 4)。
表 3 影响胰腺癌患者远期生存的单因素分析因素 β HR 95%CI P值 肿瘤大小 1.126 3.084 1.504~6.324 0.002 血管侵犯 -0.571 0.565 0.210~1.520 0.258 神经侵犯 -0.015 0.985 0.374~2.595 0.975 淋巴结转移 0.826 2.283 0.904~5.765 0.081 远处器官转移 1.719 5.577 1.472~21.135 0.011 分化程度 0.928 2.530 1.347~4.752 0.004 CA199(U/ml) 0.000 1.000 0.994~1.006 0.912 CEA(ng/ml) 0.131 1.140 1.070~1.215 <0.001 CRP(mg/dl) -0.353 0.703 0.356~1.389 0.310 ANGPTL2(ng/ml) 0.584 1.793 1.224~2.628 0.003 HbA1c(%) 0.653 1.903 1.354~2.873 0.003 表 4 影响胰腺癌患者远期生存的多因素分析因素 HR 95%CI P值 肿瘤大小 2.657 1.338~5.276 0.005 远处器官转移 5.000 1.380~18.114 0.014 分化程度 2.466 1.344~4.522 0.004 CEA 1.110 1.056~1.167 <0.001 ANGPTL2 1.901 1.310~2.759 0.001 2.5 血清ANGPTL2表达水平、是否合并糖尿病与胰腺癌患者预后的关系
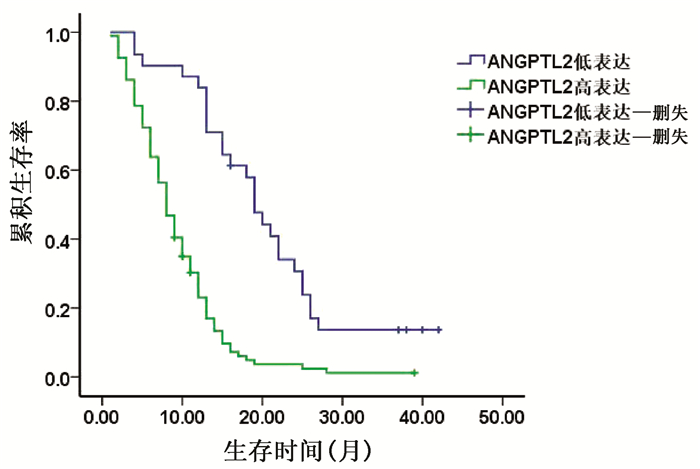
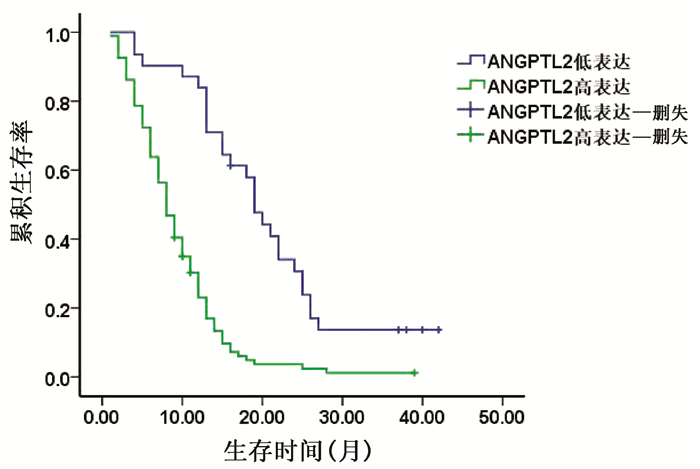
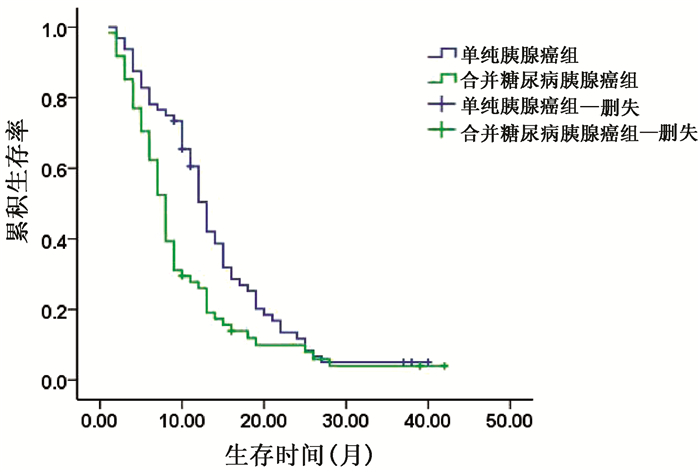
以胰腺癌患者ANGPTL2的平均值为界将其分为ANGPTL2高表达组和ANGPTL2低表达组。由于HbA1c常规参考值为(4~6)%,故本研究将HbA1c值>6%的胰腺癌患者作为合并糖尿病胰腺癌组,而HbA1c值≤6%的胰腺癌患者作为单纯胰腺癌组。结果显示,ANGPTL2高表达组的2年生存率明显低于ANGPTL2低表达组,差异有统计学意义(8.51% vs 25.81%,χ2=5.651,P=0.017)(图 3)。合并糖尿病胰腺癌组与单纯胰腺癌组相比,2年生存率差异无统计学意义(8.20% vs 12.50%,χ2=0.621,P=0.431)(表 5,图 4)。
表 5 血清ANGPTL2表达水平、是否合并糖尿病与胰腺癌患者预后的关系参数 例数 2年生存率(%) χ2值 P值 ANGPTL2 5.651 0.017 高表达组 94 8.51 低表达组 31 25.81 HbA1c 0.621 0.431 升高 61 8.20 正常 64 12.50 2.6 血清ANGPTL2表达水平与合并糖尿病的胰腺癌患者预后的关系
在合并糖尿病的胰腺癌患者中,ANGPTL2高表达组的2年生存率明显低于ANGPTL2低表达组,差异有统计学意义(2.20% vs 32.70 %,χ2=24.895,P<0.001)。
3. 讨论
文献[9]报道,恶性肿瘤独特的能量获取途径——糖酵解往往能使恶性肿瘤患者快速消耗脂肪和蛋白质,从而处于代谢异常状态;反之,不同程度的代谢异常也常常在恶性肿瘤发生发展中发挥作用,其中包括血糖异常。由于胰腺癌所处部位的特殊性,使得其与血糖的关系更为密切:一方面,肿瘤组织对胰岛的破坏和胰管的阻塞可能导致糖尿病的发生;另一方面,糖尿病可能通过多种机制促进胰腺癌的发生和发展。ANGPTL2是近年来关注度较高的生物分子。有趣的是,研究报道认为ANGPTL2可能引起胰腺癌[10]或糖尿病[11],而胰腺癌与糖尿病又互为因果[12-14]。据此笔者从临床角度探讨胰腺癌患者在合并与未合并糖尿病时其血清ANGPTL2的表达情况,进而探讨ANGPTL2的表达与胰腺癌患者,特别是合并糖尿病的胰腺癌患者预后的关系。
ANGPTL2在正常生理条件下主要分布于人类的心脏、胃、脾脏和小肠等部位,少量分布于卵巢、胰腺和胸腺组织中[5, 15]。ANGPTL2可以由多种不同细胞分泌,如脂肪细胞[15]、内皮细胞[16]、巨噬细胞[17]、角质形成细胞[18]和癌细胞[19],因此具备促进血管生成、炎症和癌症发生等多种重要生理和病理功能。已有文献报道ANGPTL2是一种促癌因子,在多种类型的恶性肿瘤患者中呈高表达,包括肺癌[20-21]、骨肉瘤[7]、甲状腺癌[22]、结肠癌[8]、胃癌[23]、白血病[24]等。胰腺癌是最致命的恶性肿瘤之一,其预后不良与早期转移行为、侵袭性临床过程和化疗效果有限有关。研究[25]表明,ANGPTL2也可能与胰腺癌的发生发展有关:ANGPTL2在胰腺癌细胞中过度表达可导致肿瘤细胞的间充质转化。另有研究[26]表明,ANGPTL2及其受体LILRB2之间的自分泌信号传导能够诱导前胰腺导管细胞模型的肿瘤进展。但是,这些基础研究结果尚待进一步的临床证实。
本研究结果显示,合并糖尿病胰腺癌组患者血清ANGPTL2水平明显高于单纯胰腺癌组,而后者ANGPTL2水平明显高于健康对照组,提示合并糖尿病的胰腺癌患者由于糖尿病和胰腺癌的双重叠加作用,其血清ANGPTL2水平可能成为比胰腺癌常用肿瘤检测指标(CA19-9、CEA)更敏感的早期诊断或预后指标。3组血清ANGPTL2与CEA、CA19-9的水平变化趋势相一致;进一步相关分析证实,血清ANGPTL2水平与CEA、CA19-9呈正相关,表明在胰腺癌的发生发展中可能存在某些相互关联的机制将三者联系起来[27]。单因素分析发现,肿瘤大小、远处器官转移、分化程度、CEA、ANGPTL2、HbA1c与胰腺癌患者远期生存密切相关,其中肿瘤大小、远处器官转移、分化程度、CEA和ANGPTL2是影响胰腺癌患者预后的独立危险因素。随后,对生存率采用log-rank检验,结果显示,在所有胰腺癌患者中ANGPTL2高表达组的2年生存率明显低于ANGPTL2低表达组,在合并糖尿病的胰腺癌患者中ANGPTL2高表达组的2年生存率也明显低于ANGPTL2低表达组;而血糖升高组胰腺癌患者与糖正常组胰腺癌患者相比,2年生存率差异无统计学意义。这可能与时间截点的选取和样本量有关,也可能与本研究的局限性有关,例如本研究的一般检验数据涉及3家医院的检验科,可能存在检测指标的室间差异。
综上所述,本研究结果提示ANGPTL2具有评估胰腺患者预后的潜力,并且在合并糖尿病的胰腺癌患者中其升高幅度更大;又由于血清ANGPTL2检测具有简便、可重复的优势,ANGPTL2可能成为评价胰腺癌患者,特别是合并糖尿病的胰腺癌患者预后的有效指标。
-
表 1 3组一般资料比较
项目 单纯胰腺癌组(n=64) 合并糖尿病胰腺癌组(n=61) 健康对照组(n=66) 统计值 P值 年龄(岁) 63.08±8.25 61.00±8.23 62.91±8.78 F=1.166 0.314 性别(男/女) 35/29 34/27 36/30 χ2=0.021 0.989 BMI(kg/m2) 24.29±1.92 23.40±2.05 25.16±2.00 F=12.371 <0.001 HbA1c(%) 5.50±0.29 8.28±0.59 5.27±0.16 F=119.6 <0.001 CRP(mg/dl) 1.61±0.68 1.85±0.621) 0.25±0.12 F=170.022 <0.001 TC(mg/dl) 178.17±22.61 167.78±22.661)2) 186.00±22.34 F=10.394 <0.001 TG(mg/dl) 119.59±28.62 122.67±26.431) 97.59±25.55 F=16.696 <0.001 注:1)与健康对照组比较,P<0.05;2)与单纯胰腺癌组比较,P<0.05。 表 2 3组人群血清CEA、CA19-9及ANGPTL2水平比较
组别 例数 CEA(ng/ml) CA19-9(U/ml) ANGPTL2(ng/ml) 单纯胰腺癌组 64 14.71(9.07~24.07) 83.11(40.85~109.77) 5.74(5.08~6.40) 合并糖尿病胰腺癌组 61 30.43(18.99~37.12) 130.45(98.62~214.01) 7.79(7.12~8.17) 健康对照组 66 2.01(1.42~2.93) 13.08(9.03~19.60) 3.72(3.25~4.16) χ2值 135.7011) 114.5181) 126.3671) P值 <0.001 <0.001 <0.001 注: 组间两两比较,1)P值均<0.05。 表 3 影响胰腺癌患者远期生存的单因素分析
因素 β HR 95%CI P值 肿瘤大小 1.126 3.084 1.504~6.324 0.002 血管侵犯 -0.571 0.565 0.210~1.520 0.258 神经侵犯 -0.015 0.985 0.374~2.595 0.975 淋巴结转移 0.826 2.283 0.904~5.765 0.081 远处器官转移 1.719 5.577 1.472~21.135 0.011 分化程度 0.928 2.530 1.347~4.752 0.004 CA199(U/ml) 0.000 1.000 0.994~1.006 0.912 CEA(ng/ml) 0.131 1.140 1.070~1.215 <0.001 CRP(mg/dl) -0.353 0.703 0.356~1.389 0.310 ANGPTL2(ng/ml) 0.584 1.793 1.224~2.628 0.003 HbA1c(%) 0.653 1.903 1.354~2.873 0.003 表 4 影响胰腺癌患者远期生存的多因素分析
因素 HR 95%CI P值 肿瘤大小 2.657 1.338~5.276 0.005 远处器官转移 5.000 1.380~18.114 0.014 分化程度 2.466 1.344~4.522 0.004 CEA 1.110 1.056~1.167 <0.001 ANGPTL2 1.901 1.310~2.759 0.001 表 5 血清ANGPTL2表达水平、是否合并糖尿病与胰腺癌患者预后的关系
参数 例数 2年生存率(%) χ2值 P值 ANGPTL2 5.651 0.017 高表达组 94 8.51 低表达组 31 25.81 HbA1c 0.621 0.431 升高 61 8.20 正常 64 12.50 -
[1] ANTOLINO L, ROCCA M, TODDE F, et al. Can pancreatic cancer be detected by adrenomedullin in patients with new-onset diabetes? The PaCANOD cohort study protocol[J]. Tumori, 2018, 104(4): 312-314. DOI: 10.5301/tj.5000693. [2] ZHANG Z, QIN W, SUN Y. Contribution of biomarkers for pancreatic cancer-associated new-onset diabetes to pancreatic cancer screening[J]. Pathol Res Pract, 2018, 214(12): 1923-1928. DOI: 10.1016/j.prp.2018.10.003. [3] RISCH HA. Diabetes and pancreatic cancer: Both cause and effect[J]. J Natl Cancer Inst, 2019, 111(1): 1-2. DOI: 10.1093/jnci/djy093. [4] KADOMATSU T, ENDO M, MIYATA K, et al. Diverse roles of ANGPTL2 in physiology and pathophysiology[J]. Trends Endocrinol Metab, 2014, 25(5): 245-254. DOI: 10.1016/j.tem.2014.03.012. [5] KIM I, KWAK HJ, AHN JE, et al. Molecular cloning and characterization of a novel angiopoietin family protein, angiopoietin-3[J]. FEBS Lett, 1999, 443(3): 353-356. DOI: 10.1016/s0014-5793(99)00008-3. [6] KENAWY MZ, SABRY JH, AKL EM, et al. Angiopoietin-like protein 2 in psoriasis: A new linkage with metabolic syndrome[J]. Postepy Dermatol Alergol, 2020, 37(1): 86-91. DOI: 10.5114/ada.2018.79225. [7] WANG X, HU Z, WANG Z, et al. Angiopoietin-like protein 2 is an important facilitator of tumor proliferation, metastasis, angiogenesis and glycolysis in osteosarcoma[J]. Am J Transl Res, 2019, 11(10): 6341-6355. http://www.ncbi.nlm.nih.gov/pubmed/31737187 [8] HE J, XU J, YU X, et al. Overexpression of ANGPTL2 and LILRB2 as predictive and therapeutic biomarkers for metastasis and prognosis in colorectal cancer[J]. Int J Clin Exp Pathol, 2018, 11(5): 2281-2294. http://www.ncbi.nlm.nih.gov/pubmed/31938340 [9] ABBASZADEH Z, ÇEŞMELI S, BIRAY AÇ. Crucial players in glycolysis: Cancer progress[J]. Gene, 2020, 726: 144158. DOI: 10.1016/j.gene.2019.144158. [10] YOSHINAGA T, NIOU T, NⅡHARA T, et al. Angiopoietin-like protein 2 is a useful biomarker for pancreatic cancer that is associated with type 2 diabetes mellitus and inflammation[J]. J Cancer, 2018, 9(24): 4736-4741. DOI: 10.7150/jca.25404. [11] WANG Y, ZHENG Z, YANG Y, et al. Angiopoietin-like 2 is a potential biomarker for diabetic foot patients[J]. BMC Endocr Disord, 2020, 20(1): 178. DOI: 10.1186/s12902-020-00657-7. [12] BOURSI B, FINKELMAN B, GIANTONIO BJ, et al. A clinical prediction model to assess risk for pancreatic cancer among patients with prediabetes[J]. Eur J Gastroenterol Hepatol, 2021. DOI: 10.1097/MEG.0000000000002052.[Online ahead of print] [13] ANDERSEN DK, KORC M, PETERSEN GM, et al. Diabetes, pancreatogenic diabetes, and pancreatic cancer[J]. Diabetes, 2017, 66(5): 1103-1110. DOI: 10.2337/db16-1477. [14] PIZZATO M, TURATI F, ROSATO V, et al. Exploring the link between diabetes and pancreatic cancer[J]. Expert Rev Anticancer Ther, 2019, 19(8): 681-687. DOI: 10.1080/14737140.2019.1642109. [15] TABATA M, KADOMATSU T, FUKUHARA S, et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance[J]. Cell Metab, 2009, 10(3): 178-188. DOI: 10.1016/j.cmet.2009.08.003. [16] FARHAT N, THORIN-TRESCASES N, MAMARBACHI M, et al. Angiopoietin-like 2 promotes atherogenesis in mice[J]. J Am Heart Assoc, 2013, 2(3): e000201. DOI: 10.1161/JAHA.113.000201. [17] TAZUME H, MIYATA K, TIAN Z, et al. Macrophage-derived angiopoietin-like protein 2 accelerates development of abdominal aortic aneurysm[J]. Arterioscler Thromb Vasc Biol, 2012, 32(6): 1400-1409. DOI: 10.1161/ATVBAHA.112.247866. [18] OGATA A, ENDO M, AOI J, et al. The role of angiopoietin-like protein 2 in pathogenesis of dermatomyositis[J]. Biochem Biophys Res Commun, 2012, 418(3): 494-499. DOI: 10.1016/j.bbrc.2012.01.052. [19] ENDO M, NAKANO M, KADOMATSU T, et al. Tumor cell-derived angiopoietin-like protein ANGPTL2 is a critical driver of metastasis[J]. Cancer Res, 2012, 72(7): 1784-1794. DOI: 10.1158/0008-5472.CAN-11-3878. [20] CHEN Y, JIANG H, ZHU L, et al. Diagnostic and prognostic value of serum angiopoietin-like protein 2 in patients with non-small cell lung cancer[J]. Clin Lab, 2017, 63(1): 59-65. DOI: 10.7754/Clin.Lab.2016.160528. [21] OSUMI H, HORIGUCHI H, KADOMATSU T, et al. Tumor cell-derived angiopoietin-like protein 2 establishes a preference for glycolytic metabolism in lung cancer cells[J]. Cancer Sci, 2020, 111(4): 1241-1253. DOI: 10.1111/cas.14337. [22] YANG L, SUN R, WANG Y, et al. Expression of ANGPTL2 and its impact on papillary thyroid cancer[J]. Cancer Cell Int, 2019, 19: 204. DOI: 10.1186/s12935-019-0908-9. [23] YOSHINAGA T, NISHIMATA H, TANAKA S, et al. Use of ANGPTL2 mRNA levels in formalin-fixed paraffin-embedded tissues as a biomarker to diagnose gastric cancer and to evaluate the extent of vascular invasion[J]. Oncol Lett, 2019, 17(1): 518-524. DOI: 10.3892/ol.2018.9610. [24] HUANG D, SUN G, HAO X, et al. ANGPTL2-containing small extracellular vesicles from vascular endothelial cells accelerate leukemia progression[J]. J Clin Invest, 2021, 131(1): e138986. DOI: 10.1172/JCI138986. [25] CARBONE C, MOCCIA T, ZHU C, et al. Anti-VEGF treatment-resistant pancreatic cancers secrete proinflammatory factors that contribute to malignant progression by inducing an EMT cell phenotype[J]. Clin Cancer Res, 2011, 17(17): 5822-5832. DOI: 10.1158/1078-0432.CCR-11-1185. [26] CARBONE C, PIRO G, FASSAN M, et al. An angiopoietin-like protein 2 autocrine signaling promotes EMT during pancreatic ductal carcinogenesis[J]. Oncotarget, 2015, 6(15): 13822-13834. DOI: 10.18632/oncotarget.2635. [27] CHEN H, LI T, WANG ZJ, et al. Influence of metabolic syndrome and its components on the prognosis of patients with pancreatic cancer[J]. J Clin Hepatol, 2020, 36(12): 2788-2794. DOI: 10.3969/j.issn.1001-5256.2020.12.029.陈欢, 李婷, 王紫洁, 等. 代谢综合征及其组分对胰腺癌患者预后的影响[J]. 临床肝胆病杂志, 2020, 36(12): 2788-2794. DOI: 10.3969/j.issn.1001-5256.2020.12.029. 期刊类型引用(2)
1. 操燕红,周康. 2型糖尿病患者血糖水平与肿瘤标志物CEA、CA199、AFP的相关性分析. 系统医学. 2023(09): 1-4 .  百度学术
百度学术2. 冷在华. ANGPTL3、Myostatin对妊娠期糖尿病新生儿低血糖的预测价值. 中华养生保健. 2023(11): 54-58 .  百度学术
百度学术其他类型引用(2)
-




 PDF下载 ( 2387 KB)
PDF下载 ( 2387 KB)

 下载:
下载:




 下载:
下载:
 百度学术
百度学术