胰腺癌组织中L1细胞黏附分子和转化生长因子β1的表达及意义
DOI: 10.3969/j.issn.1001-5256.2021.06.035
Expression and significance of L1 cell adhesion molecule and transforming growth factor-β1 in pancreatic cancer tissue
-
摘要:
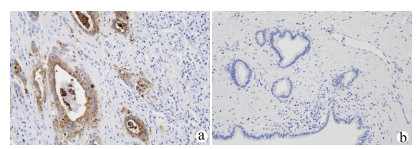
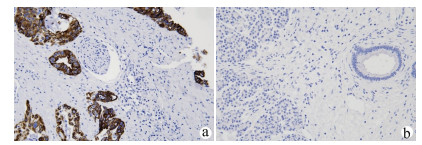
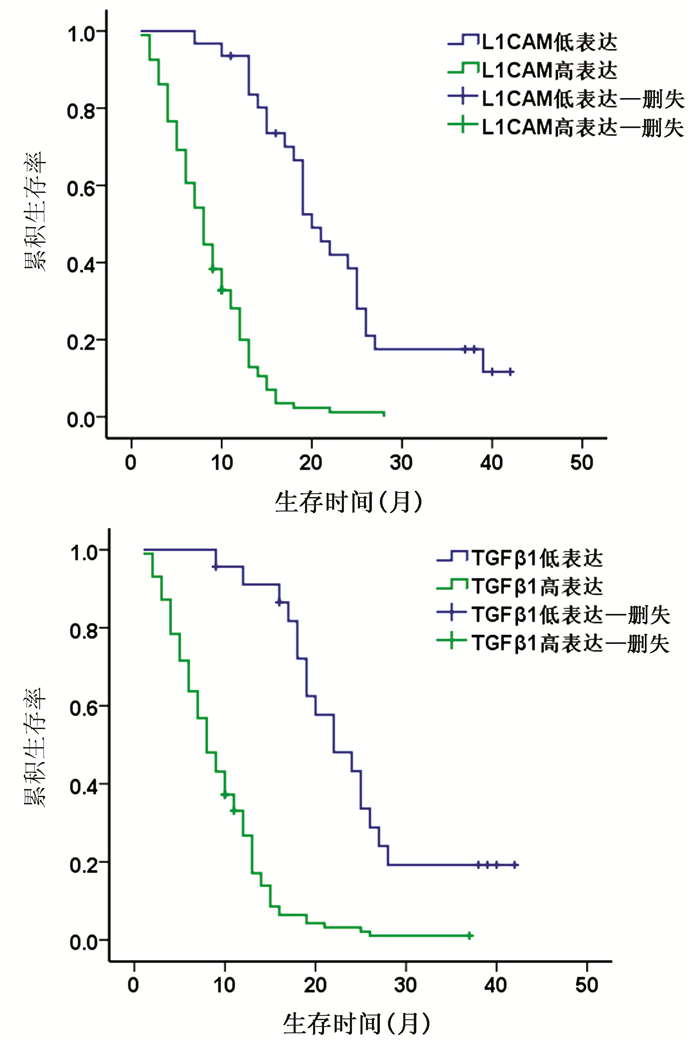
目的 探讨分析L1细胞黏附分子(L1CAM)和转化生长因子β1(TGFβ1)在胰腺癌组织中的表达及其与预后的关系。 方法 选取2015年1月—2018年1月于广西医科大学第一附属医院、广西医科大学附属肿瘤医院、广西医科大学附属武鸣医院行手术切除的125例胰腺癌患者的组织学标本。采用免疫组化法检测所有标本L1CAM(高表达组94例,低表达组31例)和TGFβ1(高表达组102例,低表达组23例)表达情况,并分析二者表达与临床指标、生存预后的关系。计量资料2组间比较采用t检验;计数资料2组间比较采用χ2检验。采用Cox风险比例模型分析胰腺癌患者生存的影响因素;Kaplan-Meier生存分析评估不同L1CAM、TGFβ1表达水平患者的生存情况,log-rank进行检验。 结果 胰腺癌组织L1CAM蛋白高表达率为75.20%,明显高于癌旁组织L1CAM蛋白高表达率(20.00%),差异有统计学意义(χ2=76.352,P<0.001)。胰腺癌组织TGFβ1蛋白高表达率为81.60%,明显高于癌旁组织TGFβ1蛋白高表达率(23.20%),差异有统计学意义(χ2=85.461,P<0.001)。L1CAM和TGFβ1蛋白在胰腺癌组织中的表达呈正相关,差异有统计学意义(r=0.492,P<0.001)。L1CAM和TGFβ1蛋白表达均与肿瘤大小、分化程度、TNM分期、淋巴结转移、脉管内癌栓和神经侵犯有关(P值均<0.05)。L1CAM或TGFβ1蛋白高表达患者总生存率明显低于低表达患者,差异均有统计学意义(χ2值分别为54.661、39.597,P值均<0.001)。 结论 L1CAM蛋白和TGFβ1蛋白在胰腺癌组织中高表达,其对不良预后的影响可能与其促进淋巴道转移、血行转移等因素有关,二者在胰腺癌发生发展及转移过程中发挥着重要作用。 Abstract:Objective To investigate the expression of L1 cell adhesion molecule (L1CAM) and transforming growth factor-β1 (TGFβ1) in pancreatic cancer tissue and their association with the prognosis of pancreatic cancer. Methods Histological specimens were collected from 125 patients with pancreatic cancer who underwent surgical resection in The First Affiliated Hospital of Guangxi Medical University, Guangxi Medical University Cancer Hospital, and Wuming Hospital of Guangxi Medical University from January 2015 to January 2018. Immunohistochemistry was used to measure the expression of L1CAM and TGFβ1 in all specimens, and the association of the expression of L1CAM and TGFβ1 with clinical indices, survival, and prognosis was analyzed. The t-test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between two groups; the Cox proportional-hazards regression model was used to investigate the influencing factors for the survival of patients with pancreatic cancer; the Kaplan-Meier survival analysis was used to evaluate the survival of patients with different expression levels of L1CAM and TGFβ1. Results The high protein expression rate of L1CAM in pancreatic cancer tissue was significantly higher than that in adjacent tissue (75.20% vs 20.00%, χ2=76.352, P < 0.001). The high protein expression rate of TGFβ1 in pancreatic cancer tissue was significantly higher than that in adjacent tissue (81.60% vs 23.20%, χ2=85.461, P < 0.001). The protein expression of L1CAM was positively correlated with that of TGFβ1 in pancreatic cancer (r=0.492, P < 0.001). The protein expression of L1CAM and TGFβ1 were associated with tumor size, degree of tumor differentiation, TNM stage, lymph node metastasis, intravascular tumor thrombus, and perineural invasion (all P < 0.05). The patients with high protein expression of L1CAM or TGFβ1 had a significantly lower overall survival rate than those with low expression (χ2=54.661 and 39.597, both P < 0.001). Conclusion L1CAM and TGFβ1 proteins are highly expressed in pancreatic cancer tissue and may be associated with poor prognosis by promoting lymphatic metastasis and hematogenous metastasis. L1CAM and TGFβ1 proteins play an important role in the development, progression, and metastasis of pancreatic cancer. -
表 1 胰腺癌组织中L1CAM和TGFβ1的表达水平与各种临床病理参数的关系
项目 L1CAM χ2值 P值 TGFβ1 χ2值 P值 高表达组(n=94) 低表达组(n=31) 高表达组(n=102) 低表达组(n=23) 性别[例(%)] 0.214 0.643 0.104 0.747 男 53(76.81) 16(23.19) 57(82.61) 12(17.39) 女 41(73.21) 15(26.79) 45(81.36) 11(19.64) 年龄[例(%)] 0.004 0.952 1.103 0.294 <60岁 40(75.47) 13(24.53) 41(77.36) 12(22.64) ≥60岁 54(75.00) 18(25.00) 61(84.72) 11(15.28) 肿瘤直径[例(%)] 61.406 <0.001 47.310 <0.001 T≤2 cm 2(8.70) 21(91.30) 6(26.09) 17(73.91) 2 cm<T≤4 cm 76(89.41) 9(10.59) 80(94.12) 5(5.88) T>4 cm 16(94.12) 1(5.88) 16(94.12) 1(5.88) 肿瘤部位[例(%)] 0.467 <0.001 0.541 0.462 胰头 61(77.22) 18(22.78) 66(83.54) 13(16.46) 胰体尾 33(71.74) 13(28.26) 36(78.26) 10(21.74) 分化程度[例(%)] 19.087 <0.001 18.693 <0.001 低分化 42(85.71) 7(14.29) 46(93.88) 3(6.12) 中分化 44(80.00) 11(20.00) 46(83.64) 9(16.36) 高分化 8(38.10) 13(61.90) 10(47.62) 11(52.38) TNM分期[例(%)] 70.378 <0.001 56.256 <0.001 Ⅰ期 2(8.70) 21(91.30) 6(26.09) 17(73.91) Ⅱ期 41(82.00) 9(18.00) 44(88.00) 6(12.00) Ⅲ期~Ⅳ期 51(98.08) 1(1.92) 52(100.00) 0 淋巴结转移[例(%)] 26.283 <0.001 19.535 <0.001 有 59(95.16) 3(4.84) 60(96.77) 2(3.23) 无 35(55.56) 28(44.44) 42(66.67) 21(33.33) 脉管内癌栓[例(%)] 18.598 <0.001 12.610 <0.001 有 54(93.10) 4(6.90) 55(94.83) 3(5.17) 无 40(59.70) 27(40.30) 47(70.15) 20(29.85) 神经侵犯[例(%)] 7.143 0.008 10.446 0.001 有 47(87.04) 7(12.96) 51(94.44) 3(5.56) 无 47(66.20) 24(33.80) 51(71.83) 20(28.17) 表 2 Cox风险回归模型分析胰腺癌患者3年生存情况与临床病理参数的关系
项目 单因素分析 多因素分析 HR P值 95%CI HR P值 95%CI 性别 0.061 0.746 1.062(0.736~1.533) 年龄 0.007 0.591 1.007(0.982~1.033) 肿瘤大小 2.103 <0.001 8.192(5.371~12.495) 0.575 0.099 1.776(0.897~3.517) 肿瘤部位 0.236 0.216 1.266(0.871~1.840) 分化程度 -0.584 <0.001 0.558(0.432~0.719) TNM分期 2.779 <0.001 16.098(10.019~25.863) 2.626 <0.001 13.823(5.281~36.184) 淋巴结转移 -2.051 <0.001 0.129(0.081~0.204) 0.338 0.363 1.402(0.677~2.899) 脉管内癌栓 -1.355 <0.001 0.258(0.171~0.390) 神经侵犯 -1.138 <0.001 0.320(0.215~0.476) 远处转移 -3.119 <0.001 0.044(0.020~0.098) 0.772 0.215 2.164(0.638~7.336) L1CAM 1.732 <0.001 5.654(3.405~9.390) 0.589 0.081 1.802(0.929~3.495) TGFβ1 1.628 <0.001 5.095(2.933~8.852) 0.702 0.053 2.019(0.991~4.113) -
[1] CHU CL, TANG CH, XU LY, et al. Association between Toll-like receptor 4 and pancreatic cancer[J]. J Clin Hepatol, 2021, 37(2): 485-488. DOI: 10.3969/j.issn.1001-5256.2021.02.050.褚成龙, 唐朝辉, 徐露瑶, 等. Toll样受体4与胰腺癌的关系[J]. 临床肝胆病杂志, 2021, 37(2): 485-488. DOI: 10.3969/j.issn.1001-5256.2021.02.050. [2] CHEN W, ZHENG R, BAADE PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2): 115-132. DOI: 10.3322/caac.21338. [3] SAMATA B, TAKAICHI R, ISHⅡ Y, et al. L1CAM is a marker for enriching corticospinal motor neurons in the developing brain[J]. Front Cell Neurosci, 2020, 14: 31. DOI: 10.3389/fncel.2020.00031. [4] LINNEBERG C, TOFT C, KJAER-SORENSEN K, et al. L1cam-mediated developmental processes of the nervous system are differentially regulated by proteolytic processing[J]. Sci Rep, 2019, 9(1): 3716. DOI: 10.1038/s41598-019-39884-x. [5] SCHÄFER MK, ALTEVOGT P. L1CAM malfunction in the nervous system and human carcinomas[J]. Cell Mol Life Sci, 2010, 67(14): 2425-2437. DOI: 10.1007/s00018-010-0339-1. [6] ALTEVOGT P, DOBERSTEIN K, FOGEL M. L1CAM in human cancer[J]. Int J Cancer, 2016, 138(7): 1565-1576. DOI: 10.1002/ijc.29658. [7] ANGIOLINI F, CAVALLARO U. The pleiotropic role of L1CAM in tumor vasculature[J]. Int J Mol Sci, 2017, 18(2): 254. DOI: 10.3390/ijms18020254. [8] KIM KK, SHEPPARD D, CHAPMAN HA. TGF-β1 signaling and tissue fibrosis[J]. Cold Spring Harb Perspect Biol, 2018, 10(4): a022293. DOI: 10.1101/cshperspect.a022293. [9] TANG J, GIFFORD CC, SAMARAKOON R, et al. Deregulation of negative controls on TGF-β1 signaling in tumor progression[J]. Cancers (Basel), 2018, 10(6): 159. DOI: 10.3390/cancers10060159. [10] LIANG M, LIU XC, LIU T, et al. GLI-1 facilitates the EMT induced by TGF-β1 in gastric cancer[J]. Eur Rev Med Pharmacol Sci, 2018, 22(20): 6809-6815. DOI: 10.26355/eurrev_201810_16148. [11] YEN EY, MIAW SC, YU JS, et al. Exosomal TGF-β1 is correlated with lymphatic metastasis of gastric cancers[J]. Am J Cancer Res, 2017, 7(11): 2199-2208. http://pubmedcentralcanada.ca/pmcc/articles/PMC5714749/ [12] MOON JR, OH SJ, LEE CK, et al. TGF-β1 protects colon tumor cells from apoptosis through XAF1 suppression[J]. Int J Oncol, 2019, 54(6): 2117-2126. DOI: 10.3892/ijo.2019.4776. [13] FEZZA M, MOUSSA M, AOUN R, et al. DKK1 promotes hepatocellular carcinoma inflammation, migration and invasion: Implication of TGF-β1[J]. PLoS One, 2019, 14(9): e0223252. DOI: 10.1371/journal.pone.0223252. [14] MARTELOSSI CEBINELLI GC, PAIVA TRUGILO K, BADARÓ GARCIA S, et al. TGF-β1 functional polymorphisms: A review[J]. Eur Cytokine Netw, 2016, 27(4): 81-89. DOI: 10.1684/ecn.2016.0382. [15] SAMATOV TR, WICKLEIN D, TONEVITSKY AG. L1CAM: Cell adhesion and more[J]. Prog Histochem Cytochem, 2016, 51(2): 25-32. DOI: 10.1016/j.proghi.2016.05.001. [16] PACE KR, DUTT R, GALILEO DS. Exosomal L1CAM stimulates glioblastoma cell motility, proliferation, and invasiveness[J]. Int J Mol Sci, 2019, 20(16): 3982. DOI: 10.3390/ijms20163982. [17] WACHOWIAK R, KRAUSE M, MAYER S, et al. Increased L1CAM (CD171) levels are associated with glioblastoma and metastatic brain tumors[J]. Medicine (Baltimore), 2018, 97(38): e12396. DOI: 10.1097/MD.0000000000012396. [18] ABDEL AZIM S, SPRUNG S, MUTZ-DEHBALAIE I, et al. L1CAM and HER2 expression in early endometrioid uterine cancer[J]. Int J Gynecol Pathol, 2017, 36(4): 356-363. DOI: 10.1097/PGP.0000000000000338. [19] GANESH K, BASNET H, KAYGUSUZ Y, et al. L1CAM defines the regenerative origin of metastasis-initiating cells in colorectal cancer[J]. Nat Cancer, 2020, 1(1): 28-45. DOI: 10.1038/s43018-019-0006-x. [20] TAMPAKIS A, TAMPAKI EC, NONNI A, et al. L1CAM expression in colorectal cancer identifies a high-risk group of patients with dismal prognosis already in early-stage disease[J]. Acta Oncol, 2020, 59(1): 55-59. DOI: 10.1080/0284186X.2019.1667022. [21] YU H, ZHOU P, LI D, et al. L1CAM-positive expression is associated with poorer survival outcomes in resected non-small cell lung cancer patients[J]. Int J Clin Exp Pathol, 2019, 12(7): 2665-2671. http://www.ncbi.nlm.nih.gov/pubmed/31934096 [22] ALTEVOGT P, BEN-ZE'EV A, GAVERT N, et al. Recent insights into the role of L1CAM in cancer initiation and progression[J]. Int J Cancer, 2020, 147(12): 3292-3296. DOI: 10.1002/ijc.33177. [23] GIORDANO M, CAVALLARO U. Different shades of L1CAM in the pathophysiology of cancer stem cells[J]. J Clin Med, 2020, 9(5): 1502. DOI: 10.3390/jcm9051502. [24] ABDEL AZIM S, SPRUNG S, MUTZ-DEHBALAIE I, et al. L1CAM and HER2 Expression in Early Endometrioid Uterine Cancer[J]. Int J Gynecol Pathol, 2017, 36(4): 356-363. DOI: 10.1097/PGP.0000000000000338. 期刊类型引用(4)
1. 王一涵,孙怀聪,裴德霖,孙诗涵,陈岚芳,王勇. 海南省肝癌风险预测模型的建立与评价. 海南医学. 2024(17): 2438-2442 .  百度学术
百度学术2. 周荃,杨丽晖,蒋芳清. 直接抗病毒药物改善HCV相关肝细胞癌患者远期预后效果的Meta分析. 临床肝胆病杂志. 2021(08): 1836-1840 .  本站查看
本站查看3. 赵小军. 红细胞体积分布宽度与血小板比值在慢性乙型肝炎患者肝纤维化评估中的临床价值. 检验医学与临床. 2021(15): 2278-2281 .  百度学术
百度学术4. 宋晓玲,顾钧,刘颖斌. 菌群紊乱对肝胆肿瘤影响的研究进展. 中华消化外科杂志. 2020(12): 1336-1340 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 2739 KB)
PDF下载 ( 2739 KB)

 下载:
下载:
 百度学术
百度学术

