成人自身免疫性肝炎临床治疗进展
DOI: 10.3969/j.issn.1001-5256.2021.06.050
Research advances in clinical treatment of adult autoimmune hepatitis
-
摘要: 自身免疫性肝炎(AIH)是一种由免疫介导的肝细胞炎症性损伤,可进展为肝硬化及终末期肝病。及时给予免疫抑制治疗可使患者获得生化缓解甚至组织学缓解,进而改善预后。但治疗过程中由药物引起的不良反应及停药后复发的现象十分常见,因此规范治疗、适时减量、及时停药对于改善患者预后至关重要。归纳总结了国内外AIH诊治指南及相关研究进展,为临床医师治疗AIH提供一定的参考。Abstract: Autoimmune hepatitis (AIH) is an immune-mediated inflammatory injury of hepatocytes, which can develop into liver cirrhosis and end-stage liver disease. Timely immunosuppressive therapy can help patients achieve biochemical remission and even histological remission and thus improve prognosis. However, adverse drug reactions during treatment and recurrence after withdrawal are commonly seen, and therefore, standard therapy, dose reduction at the right time, and timely drug withdrawal are important for improving patients' prognosis. This article summarizes the advances in guidelines for the diagnosis and treatment of AIH and related studies in China and globally, so as to provide a reference for clinicians in the treatment of AIH.
-
Key words:
- Hepatitis, Autoimmune /
- Adult /
- Therapeutics
-
表 1 AIH相关免疫抑制药物与HBV再激活风险
HBV再激活风险分组 HBV血清特征及药物使用剂量和持续时间 高风险
(HBV再激活的发生率>10%)糖皮质激素治疗≥4周
HBsAg阳性、抗-HBc阳性者,糖皮质激素不论为中剂量(10~20 mg/d)还是高剂量(>20 mg/d)中风险
(HBV再激活的发生率1%~10%)糖皮质激素治疗≥4周
HBsAg阳性、抗-HBc阳性者,糖皮质激素低剂量(<10 mg/d) HBsAg阴性、抗-HBc阳性者,糖皮质激素不论为中剂量(10~20 mg/d)还是高剂量(>20 mg/d)低风险(HBV再激活的发生率<1%) AZA
HBsAg阳性、抗-HBc阳性<1%
HBsAg阴性、抗-HBc阳性:风险远<1%
糖皮质激素治疗≤1周
HBsAg阳性、抗-HBc阳性<1%
HBsAg阴性、抗-HBc阳性:风险远<1%
糖皮质激素治疗≥4
周HBsAg阴性、抗-HBc阳性者,糖皮质激素低剂量(<10 mg/d)表 2 成人AIH患者的治疗方案(以60 kg为例)
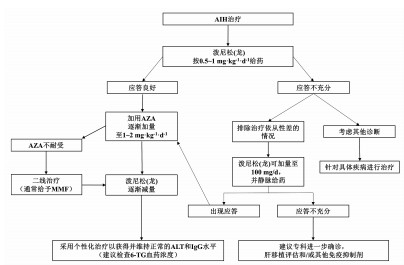
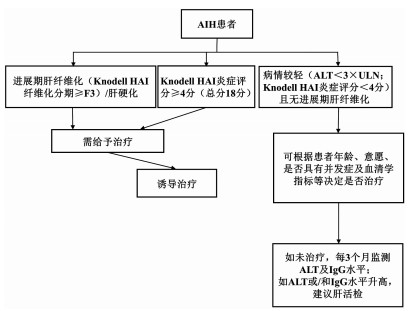
周数 泼尼松(龙)(mg/d) AZA(mg/d) 1 60(=1 mg/kg体质量) 不使用AZA 2 50 不使用AZA 3 40 50 4 30 50 5 25 1001) 6 20 1001) 7+8 15 1001) 8+9 12.5 1001) 从第10周起 10 1001) 注:如第10周后转氨酶达到正常水平,则将泼尼松(龙)继续减量至7.5 mg/d,3个月后减量至5 mg/d,并根据患者的危险因素和应答情况,泼尼松(龙)继续逐步减量,通常每3~4个月减量1次。1)AZA的剂量按1~2 mg/kg体质量。 表 3 单纯性AIH的治疗
诱导阶段 维持阶段 激素 一旦获得生化缓解 泼尼松(龙)(20~40 mg/d)
或布地奈德(9 mg/d)可尝试停用激素,改为单一AZA继续治疗
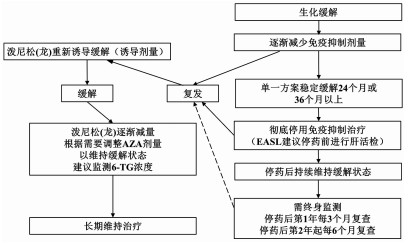
每3~4个月进行实验室监测AZA 持续生化缓解 检查TPMT,2周后增加AZA 持续24个月以上者,每4~6个月进行实验室监测 每1~2周进行实验室检查 可酌情考虑停止免疫抑制剂治疗(并非需要肝穿刺活检) 应答评估 4~8周评估应答情况 出现生化应答者: 在6个月内,泼尼松(龙)逐渐减量至5~10 mg/d
(或布地奈德减量至3 mg/d)维持使用AZA 未出现生化应答者: 重新诊断评估 考虑应用二线药物 表 4 AIH所致肝硬化的治疗
诱导阶段 维持阶段 激素 获得生化缓解者 泼尼松(龙)(20~40 mg/d) 可尝试停用激素,改为单一AZA继续治疗 不可使用布地奈德 每3~4个月进行实验室监测 AZA 持续生化缓解 不可用于失代偿期肝硬化 持续24个月以上者,每4~6个月进行实验室监测 代偿性肝硬化:需筛查TPMT 可酌情考虑停止免疫抑制剂治疗(并非需要肝穿刺活检) 泼尼松(龙)治疗2周后增加AZA(50~150 mg/d) 每1~2周进行实验室检查 应答评估 4~8周内评估应答状态 出现生化应答者: 每2~4周进行实验室检查 在6个月内,泼尼松(龙)逐渐减量至5~10 mg/d AZA一旦启动,则继续维持治疗 未出现生化应答者: 重新诊断评估 考虑应用二线药物 表 5 AIH所致肝衰竭的治疗
诱导阶段 维持阶段 激素 获得生化缓解者 泼尼松(龙)60 mg/d(口服或静脉使用均可),不可使用布地奈德及AZA 每3~4个月进行实验室检查 每12~24 h进行实验室检查 使用最低剂量免疫抑制维持缓解 应答评估 不可停用免疫抑制剂 在7~14 d评估应答 出现生化应答者: 泼尼松谨慎减量 胆汁淤积改善后考虑使用AZA(使用前应先检查TPMT) 每1~2周进行实验室检查 未出现生化应答者: 重新诊断评估 考虑二线药物 启动肝移植评估 如发生肝性脑病 紧急肝移植评估 -
[1] DANZIGER-ISAKOV L, KUMAR D, AST ID Community of Practice. Vaccination of solid organ transplant candidates and recipients: Guidelines from the American society of transplantation infectious diseases community of practice[J]. Clin Transplant, 2019, 33(9): e13563. DOI: 10.1111/ctr.13563. [2] LOOMBA R, LIANG TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: Current concepts, management strategies, and future directions[J]. Gastroenterology, 2017, 152(6): 1297-1309. DOI: 10.1053/j.gastro.2017.02.009. [3] TERRAULT NA, LOK A, McMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67(4): 1560-1599. DOI: 10.1002/hep.29800. [4] REDDY KR, BEAVERS KL, HAMMOND SP, et al. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy[J]. Gastroenterology, 2015, 148(1): 215-219. DOI: 10.1053/j.gastro.2014.10.039. [5] PERRILLO RP, GISH R, FALCK-YTTER YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy[J]. Gastroenterology, 2015, 148(1): 221-244. DOI: 10.1053/j.gastro.2014.10.038. [6] PATTULLO V. Prevention of hepatitis B reactivation in the setting of immunosuppression[J]. Clin Mol Hepatol, 2016, 22(2): 219-237. DOI: 10.3350/cmh.2016.0024. [7] EBADI M, BHANJI RA, MAZURAK VC, et al. Severe vitamin D deficiency is a prognostic biomarker in autoimmune hepatitis[J]. Aliment Pharmacol Ther, 2019, 49(2): 173-182. DOI: 10.1111/apt.15029. [8] CZAJA AJ, MONTANO-LOZA AJ. Evolving role of vitamin D in immune-mediated disease and its implications in autoimmune hepatitis[J]. Dig Dis Sci, 2019, 64(2): 324-344. DOI: 10.1007/s10620-018-5351-6. [9] American Gastroenterological Association. American Gastroenterological Association medical position statement: Osteoporosis in hepatic disorders[J]. Gastroenterology, 2003, 125(3): 937-940. DOI: 10.1016/s0016-5085(03)01060-6. [10] JANIK MK, WUNSCH E, RASZEJA-WYSZOMIRSKA J, et al. Autoimmune hepatitis exerts a profound, negative effect on health-related quality of life: A prospective, single-centre study[J]. Liver Int, 2019, 39(1): 215-221. DOI: 10.1111/liv.13960. [11] MIELI-VERGANI G, VERGANI D, CZAJA AJ, et al. Autoimmune hepatitis[J]. Nat Rev Dis Primers, 2018, 4: 18017. DOI: 10.1038/nrdp.2018.17. [12] WONG LL, FISHER HF, STOCKEN DD, et al. The impact of autoimmune hepatitis and its treatment on health utility[J]. Hepatology, 2018, 68(4): 1487-1497. DOI: 10.1002/hep.30031. [13] SHEIKO MA, SUNDARAM SS, CAPOCELLI KE, et al. Outcomes in pediatric autoimmune hepatitis and significance of azathioprine metabolites[J]. J Pediatr Gastroenterol Nutr, 2017, 65(1): 80-85. DOI: 10.1097/MPG.0000000000001563. [14] MANNS MP, WOYNAROWSKI M, KREISEL W, et al. Budesonide induces remission more effectively than prednisone in a controlled trial of patients with autoimmune hepatitis[J]. Gastroenterology, 2010, 139(4): 1198-1206. DOI: 10.1053/j.gastro.2010.06.046. [15] HEMPFLING W, GRUNHAGE F, DILGER K, et al. Pharmacokinetics and pharmacodynamic action of budesonide in early- and late-stage primary biliary cirrhosis[J]. Hepatology, 2003, 38(1): 196-202. DOI: 10.1053/jhep.2003.50266. [16] PEISELER M, LIEBSCHER T, SEBODE M, et al. Efficacy and limitations of budesonide as a second-line treatment for patients with autoimmune hepatitis[J]. Clin Gastroenterol Hepatol, 2018, 16(2): 260-267. DOI: 10.1016/j.cgh.2016.12.040. [17] ZACHOU K, GATSELIS NK, ARVANITI P, et al. A real-world study focused on the long-term efficacy of mycophenolate mofetil as first-line treatment of autoimmune hepatitis[J]. Aliment Pharmacol Ther, 2016, 43(10): 1035-1047. DOI: 10.1111/apt.13584. [18] NASTASIO S, SCIVERES M, MATARAZZO L, et al. Long-term follow-up of children and young adults with autoimmune hepatitis treated with cyclosporine[J]. Dig Liver Dis, 2019, 51(5): 712-718. DOI: 10.1016/j.dld.2018.10.018. [19] VAN THIEL DH, WRIGHT H, CARROLL P, et al. Tacrolimus: A potential new treatment for autoimmune chronic active hepatitis: Results of an open-label preliminary trial[J]. Am J Gastroenterol, 1995, 90(5): 771-776. [20] RAHIM MN, LIBERAL R, MIQUEL R, et al. Acute severe autoimmune hepatitis: Corticosteroids or liver transplantation?[J]. Liver Transpl, 2019, 25(6): 946-959. DOI: 10.1002/lt.25451. [21] CZAJA AJ, CARPENTER HA. Autoimmune hepatitis overlap syndromes and liver pathology[J]. Gastroenterol Clin North Am, 2017, 46(2): 345-364. DOI: 10.1016/j.gtc.2017.01.008. [22] KUIPER EM, ZONDERVAN PE, van BUUREN HR. Paris criteria are effective in diagnosis of primary biliary cirrhosis and autoimmune hepatitis overlap syndrome[J]. Clin Gastroenterol Hepatol, 2010, 8(6): 530-534. DOI: 10.1016/j.cgh.2010.03.004. [23] TRAN TT, AHN J, REAU NS. Corrigendum: ACG Clinical Guideline: Liver disease and pregnancy[J]. Am J Gastroenterol, 2016, 111(11): 1668. DOI: 10.1038/ajg.2016.462. [24] AKBARI M, SHAH S, VELAYOS FS, et al. Systematic review and meta-analysis on the effects of thiopurines on birth outcomes from female and male patients with inflammatory bowel disease[J]. Inflamm Bowel Dis, 2013, 19(1): 15-22. DOI: 10.1002/ibd.22948. [25] COSCIA LA, ARMENTI DP, KING RW, et al. Update on the teratogenicity of maternal mycophenolate mofetil[J]. J Pediatr Genet, 2015, 4(2): 42-55. DOI: 10.1055/s-0035-1556743. [26] TAUBERT R, HARDTKE-WOLENSKI M, NOYAN F, et al. Hyperferritinemia and hypergammaglobulinemia predict the treatment response to standard therapy in autoimmune hepatitis[J]. PLoS One, 2017, 12(6): e0179074. DOI: 10.1371/journal.pone.0179074. [27] HARTL J, EHLKEN H, WEILER-NORMANN C, et al. Patient selection based on treatment duration and liver biochemistry increases success rates after treatment withdrawal in autoimmune hepatitis[J]. J Hepatol, 2015, 62(3): 642-646. DOI: 10.1016/j.jhep.2014.10.018. [28] GUIRGUIS J, ALONSO Y, LOPEZ R, et al. Well-controlled autoimmune hepatitis treatment withdrawal may be safely accomplished without liver-biopsy guidance[J]. Gastroenterol Rep (Oxf), 2018, 6(4): 284-290. DOI: 10.1093/gastro/goy020. [29] THAN NN, WIEGARD C, WEILER-NORMANN C, et al. Long-term follow-up of patients with difficult to treat type 1 autoimmune hepatitis on Tacrolimus therapy[J]. Scand J Gastroenterol, 2016, 51(3): 329-336. DOI: 10.3109/00365521.2015.1095351. [30] HVBENER S, OO YH, THAN NN, et al. Efficacy of 6-mercaptopurine as second-line treatment for patients with autoimmune hepatitis and azathioprine intolerance[J]. Clin Gastroenterol Hepatol, 2016, 14(3): 445-453. DOI: 10.1016/j.cgh.2015.09.037. [31] MACK CL, ADAMS D, ASSIS DN, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 Practice Guidance and Guidelines from the American Association for the Study of Liver Diseases[J]. Hepatology, 2020, 72(2): 671-722. DOI: 10.1002/hep.31065. -



 PDF下载 ( 2843 KB)
PDF下载 ( 2843 KB)

 下载:
下载: