病毒学应答状态对代偿期乙型肝炎肝硬化患者疾病进展的影响
DOI: 10.3969/j.issn.1001-5256.2021.08.014
Influence of virologic response on disease progression in patients with compensated hepatitis B cirrhosis
-
摘要:
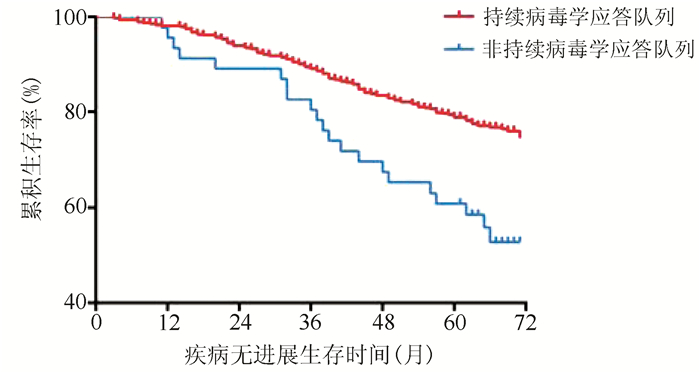
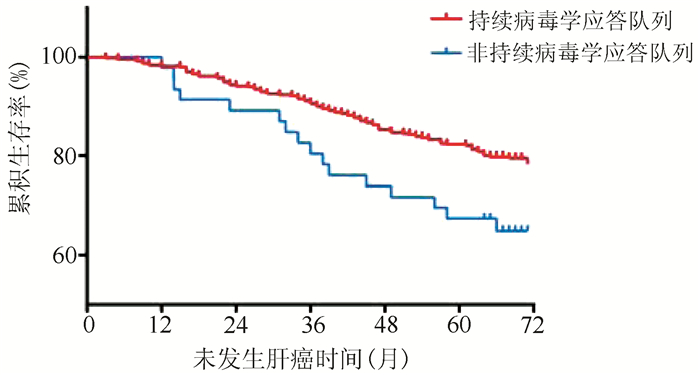
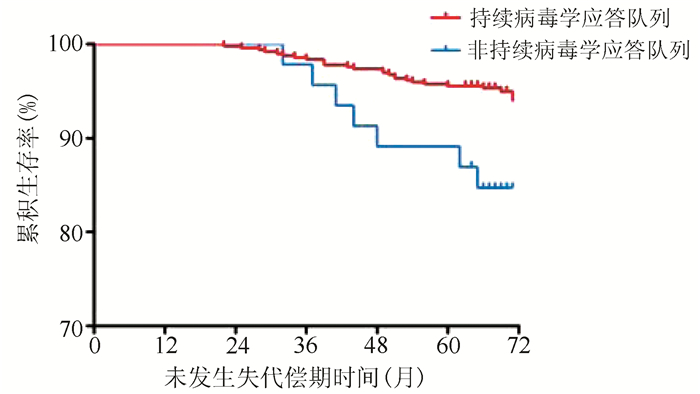
目的 研究是否获得持续性病毒学应答对接受核苷(酸)类似物(NAs)抗病毒治疗的代偿期乙型肝炎肝硬化患者疾病进展、肝癌发生的影响。 方法 纳入2013年1月1日—12月31日就诊于上海中医药大学附属曙光医院,随访5年以上、接受抗病毒治疗的代偿期乙型肝炎肝硬化患者542例,依据随访期间病毒学应答状态分为持续病毒学应答队列(n=496)和非持续病毒学应答队列(n=46),以患者疾病进展为终点事件,收集、整理患者5年随访期间一般资料及检验检查资料。符合正态分布的计量资料两组间比较采用t检验;不服从正态分布的计量资料两组间比较采用Mann-Whitney U检验。计数资料两组间比较采用χ2检验。多元分析采用logistic回归分析。用危险度测量因素与肝硬化疾病进展的相关程度,用风险比(HR)及95%CI表示。应用寿命表法计算患者1、3、5年疾病无进展生存率,Kaplan-Meier法绘制生存曲线;采用log-rank检验进行单因素分析,Cox模型进行多因素回归分析。 结果 542例患者的平均疾病无进展生存时间为62.50(95%CI:61.01~63.92)个月,1、3、5年疾病无进展生存率分别为94%、82%、71%。持续病毒学应答队列患者平均疾病无进展生存时间为63.10(95%CI:61.65~64.55)个月,较非持续病毒学应答队列[55.95(95%CI :50.19~61.71)个月]延长,两组5年疾病无进展生存率差异有统计学意义(χ2=12.058,P=0.001)。持续病毒学应答队列患者5年累积肝癌发生率低,两组差异有统计学意义(20.6% vs 34.8%,χ2=5.759,P=0.016);持续病毒学应答队列患者5年累积肝硬化失代偿发生率低,两组差异有统计学意义(5.0% vs 15.2%,χ2=8.239,P=0.004)。病毒学应答是影响疾病进展的独立危险因素[HR(95%CI)=2.32(1.45~3.72)]。 结论 持续病毒学应答可降低代偿期乙型肝炎肝硬化患者并发症及肝癌发生率,改善长期预后、延长生存时间。 Abstract:Objective To investigate the effect of sustained virologic response on disease progression and the development of hepatocellular carcinoma (HCC) in patients with compensated hepatitis B cirrhosis receiving antiviral therapy with nucleos(t)ide analogues (NAs). Methods A total of 542 patients with compensated hepatitis B cirrhosis who attended Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine from January 1 to December 31, 2013, received antiviral therapy, and were followed up for more than 5 years were enrolled, and according to the status of virologic response during follow-up, they were divided into a sustained virologic response cohort with 496 cases and a non-sustained virologic response cohort with 46 cases. With disease progression as the outcome event, general information and examination data were collected during the 5-year follow-up period. The t-test was used for comparison of normally distributed continuous data between groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between groups; the chi-square test was used for comparison of categorical data between groups. A multivariate logistic regression analysis was performed; relative risk and 95% confidence interval (CI) were used to investigate the degree of correlation of factors measured with the progression of liver cirrhosis. The life-table method was used to calculate the 1-, 3-, and 5-year progression-free survival rates, and the Kaplan-Meier method was used to plot survival curves; the log-rank test was used for univariate analysis, and the Cox regression model was used for multivariate regression analysis. Results For the 542 patients, the mean progression-free survival time was 62.50 months (95% CI: 61.01-63.92), and the 1-, 3-, and 5-year progression-free survival rates were 94%, 82%, and 71%, respectively. The sustained virologic response cohort had a significantly longer mean progression-free survival time than the non-sustained virologic response cohort [63.10 months (95% CI: 61.65-64.55) vs 55.95 months (95% CI: 50.19-61.71), χ2=12.058, P=0.001]. Compared with the non-sustained virologic response cohort, the sustained virologic response cohort had significantly lower 5-year cumulative incidence rate of HCC than (20.6% vs 34.8%, χ2=5.759, P=0.016) and 5-year cumulative incidence rate of decompensated cirrhosis (5.0% vs 15.2%, χ2=8.239, P=0.004). Virologic response was an independent risk factor for disease progression (hazard ratio=2.32, 95% CI: 1.45-3.72). Conclusion Sustained virologic response can reduce the incidence rates of complications and HCC, improve long-term prognosis, and prolong survival time in patients with compensated hepatitis B cirrhosis. -
Key words:
- Hepatitis B /
- Liver Cirrhosis /
- Sustained Virologic Response
-
非酒精性脂肪性肝病(NAFLD)是一种常见的慢性肝病,包括非酒精性脂肪肝、非酒精性脂肪性肝炎(NASH)及其相关肝纤维化和肝硬化[1],部分患者甚至进展为肝癌,现在被称为代谢相关脂肪性肝病(metabolic associated fatty liver disease,MAFLD)[2-3],随着肥胖发病率逐年增高和低龄化趋势,NAFLD成为儿童慢性肝病的常见原因[4],肝硬化的三大主要原因之一。目前MAFLD的发病机制仍不清楚,临床上亦缺乏有效的药物治疗,因此MAFLD发病机制的研究是当前的热点。
黏膜相关恒定T(mucosal associated invariant T,MAIT)淋巴细胞是一类新的天然免疫T淋巴细胞,是最丰富的TCRαβ+T淋巴细胞,以1类主要组织相容性复合体相关分子和不依赖MR1的方式快速激活MAIT淋巴细胞[5],发挥生物学功能,释放多种细胞因子,如IFNγ、TNFα、IL-17和溶细胞产物穿孔素及颗粒素分泌并脱颗粒(将CD107a暴露于细胞表面)等杀伤性细胞因子[6],迅速诱导细胞溶解和靶细胞的死亡。国外相关报道[7-9]显示MAIT淋巴细胞在成人MAFLD发病中起到减少肝脏炎症及促肝纤维化作用,但在儿童MAFLD发病中作用的研究甚少。本研究通过分析MAFLD儿童外周血MAIT淋巴细胞的变化及其与临床指标的相关性,探讨MAIT淋巴细胞在儿童MAFLD发生发展中的作用。
1. 资料与方法
1.1 研究对象
收集2022年3月—2022年5月在本院肝病中心诊治的18例MAFLD患儿(MAFLD组)的外周血标本,根据年龄匹配的20例正常儿童作为对照(对照组),其外周血标本采集于本院健康管理中心。
1.2 纳入标准
MAFLD组纳入标准:脂肪肝合并超重/肥胖、2型糖尿病和代谢功能障碍中至少一项特征[1-2]。同时排除合并甲、乙、丙、丁、戊型肝炎病毒感染,自身免疫性肝病,人类免疫缺陷病毒等病毒感染。对照组纳入标准:肝功能正常,无慢性疾病,近期无感染史等。
1.3 方法
人外周血免疫细胞的制备:留取MAFLD组和对照组儿童外周血3 mL,将PBS加入3 mL新鲜全血中,吸取稀释后的外周血缓慢加到lymphoprep表面。全血在400×g室温下离心20 min,收集第二层外周血免疫细胞并转入含有PBS的离心管中,离心机800×g室温下离心10 min。离心结束后去上清,再加入10 mL PBS,离心机400×g室温下离心5 min,清洗外周血免疫细胞以彻底去除残留的lymphoprep。
应用流式细胞仪检测MAIT细胞,根据标准方案使用以下抗体:CD183(CXCR3)、CD279(PD-1), CD186(CXCR6)、CD3、TCRVα7.2、CD8、CD69、CD161、CD107a、CD196 (CCR6)、CD4和穿孔素抗体进行染色,上流式细胞仪(三激光八色流式细胞分析仪,型号:FacsCantoll,产地:美国)进行分析。外周血中MAIT细胞定义为CD3+CD161+TCRVα7.2+细胞。
收集MAFLD组和对照组儿童血常规及肝功能结果,使用瞬时弹性成像检测MAFLD组患儿的肝纤维化和脂肪含量,儿童MAFLD肝纤维化最佳临界值为6.65 kPa[10],区分有无脂肪变性的脂肪肝参数最佳临界值为222.5 dB/m[11]。
1.4 统计学方法
采用SPSS 23.0软件进行统计学分析。符合正态分布的计量资料以x±s表示,两组间比较采用独立样本t检验;非正态分布的计量资料以M(P25~P75)表示,两组间比较采用Mann-Whitney U检验。MAFLD组患儿外周血MAIT淋巴细胞频率与肝损伤、肝脏脂肪含量和纤维化相关性分析应用Spearman相关分析法。P<0.05为差异具有统计学意义。
2. 结果
2.1 一般资料
MAFLD组18例患儿年龄波动在6.17~13.08岁,对照组20例儿童年龄波动在5.83~13.00岁。MAFLD组的中性粒细胞、ALT、AST水平高于对照组(P值均<0.05)(表 1)。MAFLD组患儿肝纤维化弹性值为5.55(4.18~7.13)kPa,脂肪肝参数为(253.85±16.40) dB/m。
表 1 两组患儿临床资料比较Table 1. The comparation of clinical data between the two groups指标 MAFLD组(n=18) 对照组(n=20) 统计值 P值 男/女(例) 10/8 11/9 年龄(岁) 10.65(8.44~11.87) 9.00(7.40~11.25) Z=-1.083 0.279 ALT(U/L) 37.30(25.08~157.53) 13.65(11.75~17.00) Z=-4.474 <0.001 AST(U/L) 29.95(26.08~62.70) 20.95(18.90~24.23) Z=-3.304 0.001 WBC(×109/L) 7.31±2.56 7.00±1.50 t=0.937 0.355 中性粒细胞(×109/L) 4.47(3.36~5.18) 3.62(2.77~3.97) Z=-2.222 0.026 淋巴细胞(×109/L) 2.76(2.38~3.11) 2.44(1.82~2.91) Z=-1.608 0.112 2.2 儿童外周血MAIT淋巴细胞频率比较
与对照组相比,MAFLD组患儿外周血MAIT淋巴细胞占CD3+T淋巴细胞的比例明显升高(P<0.001),CD4+CD8-MAIT淋巴细胞、CD4+CD8+MAIT淋巴细胞所占MAIT淋巴细胞比例明显升高,CD4-CD8+MAIT淋巴细胞所占MAIT淋巴细胞比例降低(P值均<0.001),CD4-CD8-MAIT淋巴细胞所占MAIT淋巴细胞比例无变化(P>0.05)(表 2)。
表 2 两组患儿外周血MAIT淋巴细胞及各亚组MAIT淋巴细胞频率比较Table 2. The comparation of the peripheral blood MAIT lymphocytes and subtypes between the two groups指标 MAFLD组(n=18) 对照组(n=20) Z值 P值 MAIT淋巴细胞(%) 2.98(1.83~5.99) 0.29(0.09~0.69) -4.765 <0.001 CD4+CD8-MAIT淋巴细胞(%) 13.75(3.28~30.33) 0.38(0~1.55) -3.703 <0.001 CD4-CD8-MAIT淋巴细胞(%) 26.45(1.03~47.03) 31.25(24.05~50.33) 0.254 >0.05 CD4-CD8+MAIT淋巴细胞(%) 9.20(1.16~16.80) 43.65(29.65~64.98) -3.876 <0.001 CD4+CD8+MAIT淋巴细胞(%) 25.65(14.78~84.95) 9.36(6.04~22.45) -2.675 <0.001 2.3 外周血MAIT淋巴细胞表型和功能的差异
与对照组相比,MAFLD组患儿外周血表达PD-1、CD69、CD107α、CXCR3、CXCR6和CCR6的MAIT淋巴细胞比例均明显升高(P值均<0.05)(表 3)。
表 3 两组患儿外周血MAIT淋巴细胞表型和功能的差异Table 3. Differences in phenotype and function of peripheral blood MAIT lymphocytes between the two groups指标 MAFLD组(n=18) 对照组(n=20) Z值 P值 PD-1(%) 21.80(8.38~51.70) 4.43(2.07~11.29) -3.334 0.001 CD69(%) 56.00(28.93~68.35) 18.90(13.23~31.18) -2.646 0.008 穿孔素(%) 9.32(4.53~18.80) 4.16(1.35~9.53) -1.827 0.068 CD107α(%) 45.20(26.03~59.03) 10.95(6.80~17.18) -3.172 0.002 CXCR3(%) 87.35(80.08~96.03) 44.80(32.93~53.00) -4.678 <0.001 CXCR6(%) 30.40(8.24~54.90) 6.02(3.27~21.58) -2.939 0.003 CCR6(%) 26.80(13.03~77.70) 4.94(3.32~17.13) -2.749 0.006 2.4 MAFLD组患儿外周血MAIT淋巴细胞频率与肝脏炎症、脂肪含量和纤维化程度的关系
CD4+CD8+MAIT淋巴细胞和CD107α阳性MAIT淋巴细胞比例与ALT呈负相关(P值均<0.05);MAIT淋巴细胞、CD4+CD8-MAIT淋巴细胞、CD4-CD8+MAIT淋巴细胞比例与ALT无相关性(P值均>0.05);MAIT淋巴细胞、CD4+CD8-MAIT淋巴细胞、CD4-CD8+MAIT淋巴细胞、CD4+CD8+MAIT淋巴细胞和CD107α阳性MAIT淋巴细胞比例与AST、瞬时弹性成像中脂肪肝参数以及弹性值均无相关性(P值均>0.05)(表 4)。
表 4 MAFLD组患儿外周血MAIT细胞频率与肝脏炎症、脂肪含量和纤维化程度相关性分析Table 4. Correlation analysis between the frequency of MAIT lymphocytes in peripheral blood and the degree of liver inflammation, fat content, and fibrosis in children with MAFLD项目 ALT AST 弹性值 脂肪肝参数 MAIT淋巴细胞 r值 0.041 -1.050 0.064 0.112 P值 0.871 0.677 0.801 0.660 CD4+CD8-MAIT淋巴细胞 r值 -0.305 -0.330 -0.303 -0.272 P值 0.218 0.181 0.221 0.275 CD4-CD8+MAIT淋巴细胞 r值 0.140 -0.130 -0.047 0.228 P值 0.580 0.606 0.854 0.363 CD4+CD8+MAIT淋巴细胞 r值 -0.474 -0.436 -0.347 -0.115 P值 0.047 0.071 0.158 0.650 CD107α阳性MAIT淋巴细胞 r值 -0.550 -0.334 -0.201 -0.168 P值 0.018 0.176 0.432 0.504 3. 讨论
MAIT细胞与代谢功能障碍的发展有关, 肥胖儿童外周血MAIT细胞频率高于非肥胖儿童[12],其频率随着肥胖人群年龄的增长而下降,糖尿病和肥胖成人外周血MAIT细胞频率降低[13-15],肥胖成人和儿童中的高IL-17+表型在胰岛素抵抗发展中发挥着重要作用[12, 16]。目前MAIT淋巴细胞在MAFLD发病中作用机制的研究主要集中在成人,研究发现,MAFLD患者中循环MAIT淋巴细胞频率降低[7-9, 17],伴随着CXCR6表达的增加,而肝脏中MAIT淋巴细胞数量则明显增加,与MAFLD活动评分呈正相关[8]。本研究发现,MAFLD患儿外周血MAIT淋巴细胞频率明显升高的同时伴有趋化因子CCR6、CXCR6、CXCR3表达的增加,表明MAFLD患儿外周血MAIT淋巴细胞亦具有更强的迁移至肝脏的倾向[8],MAFLD患者外周血MAIT淋巴细胞频率的变化亦有着随着年龄增长而有下降的趋势,而这种下降的趋势可能与其迁移至肝脏有关。MAIT淋巴细胞可分为DP(CD4+CD8+)MAIT淋巴细胞、CD4+CD8-(CD4+)MAIT淋巴细胞、CD4-CD8+(CD8+)MAIT淋巴细胞和CD4-CD8-(DN)MAIT淋巴细胞。本研究发现健康儿童中以CD4-CD8-MAIT淋巴细胞和CD8+MAIT淋巴细胞为主[18],而MAFLD组患儿中以CD4-CD8-MAIT淋巴细胞和CD4+CD8+MAIT淋巴细胞为主,且与对照组相比,MAFLD组患儿CD4+MAIT淋巴细胞和CD4+CD8+MAIT淋巴细胞所占MAIT细胞比率升高,CD8+MAIT淋巴细胞所占MAIT淋巴细胞比率降低。
MAIT淋巴细胞在MAFLD中的作用为保护还是促进作用,与其所处疾病阶段有关。MAFLD成人患者中,外周血MAIT淋巴细胞降低的同时伴随着功能的改变,高表达CD69、PD-1及IL-4的产生增加,而IFNγ和TNFα的产生减少等。在蛋氨酸胆碱缺乏诱导的NASH模型中,MAIT细胞在肝脏中富集,并通过产生IL-4和IL-10以及诱导抗炎M2巨噬细胞来保护肝脏炎症[8]。随着病情的进展,肝内MAIT淋巴细胞的频率下降,在肝硬化和肝细胞癌的进展中起到促进作用[7, 17, 19]。本研究发现,MAFLD患儿外周血MAIT淋巴细胞水平明显升高的同时,伴有CD69、PD-1及CD107表达的增加,提示MAIT淋巴细胞被激活并功能性耗竭的同时,伴随着细胞毒性能力的增加。进一步研究显示CD4+CD8+MAIT淋巴细胞、CD107阳性MAIT淋巴细胞比例与ALT水平呈负相关,说明MAFLD患儿外周血MAIT细胞在肝脏炎症中具有保护作用,与文献[8]报道一致。
综上所述,MAIT淋巴细胞比例在MAFLD患儿外周血中明显升高,并在肝脏炎症中起着保护作用。本研究的局限性在于样本量偏少,进展期肝纤维化患儿病例数少,未检测肝脏组织MAIT淋巴细胞数量和其功能改变,MAIT淋巴细胞在儿童MAFLD发病中确切的免疫学机制及作用尚待进一步明确。
-
表 1 持续病毒学应答队列与非持续病毒学应答队列患者基线资料比较
指标 所有患者(n=542) 持续病毒学应答队列(n=496) 非持续病毒学应答队列(n=46) 统计值 P值 男/女(例) 376/166 341/155 35/11 χ2=1.067 0.302 年龄(岁) 51.90±10.39 52.10±10.38 50.20±10.37 t=1.155 0.249 饮酒史[例(%)] 30(5.5) 26(5.2) 4(8.7) χ2=0.960 0.327 肝病家族史[例(%)] 221(40.8) 203(40.9) 18(39.1) χ2=0.056 0.812 糖尿病史[例(%)] 47(8.7) 40(8.1) 7(15.2) χ2=2.720 0.099 HBeAg阳性[例(%)] 134(24.7) 118(23.8) 16(34.8) χ2=2.733 0.098 ALT(U/L) 31.30±18.82 30.10±14.91 44.10±40.38 t=-4.926 <0.001 TBil(μmol/L) 21.60±11.53 21.60±11.62 21.40±10.62 t= 0.072 0.943 Alb(g/L) 43.30±5.59 43.30±5.46 42.20±6.88 t=1.403 0.161 SCr(μmol/L) 68.00±14.27 68.00±14.44 67.20±12.33 t=0.388 0.698 PLT(×109/L) 111.50±60.12 112.60±61.13 99.90±46.85 t=1.367 0.172 INR 1.12±0.17 1.10±0.17 1.10±0.10 t=0.492 0.623 AFP(ng/ml) 51(9.4) 43(8.7) 8(17.4) χ2=3.757 0.053 MPV(mm) 12.00±1.37 12.00±1.38 12.00±1.33 t=0.086 0.932 MELD评分 5.30±3.33 5.40±3.35 5.10±3.13 t=0.463 0.644 APRI≥2[例(%)] 70(12.9) 61(12.3) 9(19.6) χ2=1.976 0.160 FIB-4≥3.25[例(%)] 242(44.6) 221(44.6) 21(45.7) χ2=0.020 0.886 注:MPV, 门静脉宽度。 表 2 病毒学应答对患者疾病无进展生存时间影响
组别 例数 疾病无进展[例(%)] 疾病无进展生存时间 平均时间(月) 标准误 95%CI 持续病毒学应答队列 496 379(76.4) 63.10 0.740 61.65~64.55 非持续病毒学应答队列 46 25(54.3) 55.95 2.939 50.19~61.71 合计 542 404(74.5) 62.50 0.726 61.07~63.92 表 3 病毒学应答对患者未发生肝癌生存时间影响
组别 例数 未发生肝癌[例(%)] 未发生肝癌生存时间 平均时间(月) 标准误 95%CI 持续病毒学应答队列 496 394(79.4) 64.12 0.704 62.74~65.50 非持续病毒学应答队列 46 30(65.2) 58.51 2.888 52.84~64.17 合计 542 424(78.2) 63.65 0.693 62.29~65.00 表 4 病毒学应答对患者未发生失代偿生存时间影响
组别 例数 未发生失代偿[例(%)] 未发生失代偿生存时间 平均时间(月) 标准误 95%CI 持续病毒学应答队列 496 471(95.0) 69.67 0.297 69.09~70.26 非持续病毒学应答队列 46 39(84.8) 67.34 1.432 64.54~70.15 合计 542 510(94.1) 63.65 0.693 68.89~70.06 表 5 542例患者疾病进展Cox多因素分析
影响因素 回归系数 标准误 Wald P值 HR 95%CI 性别 -0.481 0.202 5.697 0.017 0.62 0.42~0.92 年龄 0.040 0.009 17.677 <0.001 1.04 1.02~1.06 肝病家族史 0.363 0.179 4.118 0.042 1.44 1.01~2.04 Alb 0.913 0.200 20.749 <0.001 2.49 1.68~3.69 PLT 0.492 0.189 6.752 0.009 1.64 1.13~2.37 AFP 1.047 0.228 21.061 <0.001 2.85 1.82~4.45 病毒学应答 0.841 0.241 12.184 <0.001 2.32 1.45~3.72 表 6 496例持续病毒学应答患者疾病进展Cox多因素分析
影响因素 回归系数 标准误 Wald P值 HR 95%CI 性别 -0.445 0.202 4.861 0.037 0.64 0.43~0.95 年龄 0.669 0.191 12.240 <0.001 1.95 1.34~2.84 肝病家族史 0.337 0.177 3.629 0.057 1.40 0.99~1.98 Alb 0.933 0.199 22.027 <0.001 2.54 1.72~3.75 PLT 0.547 0.190 8.293 0.004 1.73 1.19~2.51 AFP 1.118 0.222 25.254 <0.001 3.06 1.98~4.73 -
[1] Chinese Society of Infectious Diseases and Chinese Society of Hepatology, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B: A 2019 update[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [2] FATTOVICH G, BORTOLOTTI F, DONATO F. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factors[J]. J Hepatol, 2008, 48(2): 335-352. DOI: 10.1016/j.jhep.2007.11.011. [3] HOU JL, ZHAO W, LEE C, et al. Outcomes of long-term treatment of chronic HBV infection with entecavir or other agents from a randomized trial in 24 countries[J]. Clin Gastroenterol Hepatol, 2020, 18(2): 457-467.e21. DOI: 10.1016/j.cgh.2019.07.010. [4] Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B: A 2010 update[J]. J Clin Hepatol, 2011, 27(1): 113-128. http://lcgdbzz.org/article/id/LCGD201101035中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2010年版)[J]. 临床肝胆病杂志, 2011, 27(1): 113-128. http://lcgdbzz.org/article/id/LCGD201101035 [5] Ministry of Health of the People's Republic of China. Diagnosis, management, and treatment of hepatocellular carcinoma (V2011)[J]. J Clin Hepatol, 2011, 27(11): 1141-1159. DOI: 10.3969/j.issn.1001-5256.2011.11.004.中华人民共和国卫生部. 原发性肝癌诊疗规范(2011年版)[J]. 临床肝胆病杂志, 2011, 27(11): 1141-1159. DOI: 10.3969/j.issn.1001-5256.2011.11.004. [6] WANG FS, FAN JG, ZHANG Z, et al. The global burden of liver disease: The major impact of China[J]. Hepatology, 2014, 60(6): 2099-2108. DOI: 10.1002/hep.27406. [7] CHEN YC, CHU CM, YEH CT, et al. Natural course following the onset of cirrhosis in patients with chronic hepatitis B: A long-term follow-up study[J]. Hepatol Int, 2007, 1(1): 267-273. DOI: 10.1007/s12072-007-5001-0. [8] ZHANG Y. The analysis of the risk factors for hepatocellular carcinoma in patients with hepatitis B virus cirrhosis receiving antiviral treatment[D]. Shenyang: China Medical University, 2018.张瑶. 接受抗病毒治疗的乙肝肝硬化患者进展为肝癌的危险因素分析[D]. 沈阳: 中国医科大学, 2018. [9] CHEN CJ, YANG HI, SU J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level[J]. JAMA, 2006, 295(1): 65-73. DOI: 10.1001/jama.295.1.65. [10] ZHANG MJ. Analysis and discussion on the effect of antiviral therapy for patients with decompensated cirrhosis of hepatitis B[J]. China Prac Med, 2018, 13(25): 5-7. DOI: 10.14163/j.cnki.11-5547/r.2018.25.003.张敏杰. 乙型肝炎肝硬化失代偿期患者抗病毒治疗效果分析与探讨[J]. 中国实用医药, 2018, 13(25): 5-7. DOI: 10.14163/j.cnki.11-5547/r.2018.25.003. [11] NAM JY, CHANG Y, CHO H, et al. Delayed viral suppression during antiviral therapy is associated with increased hepatocellular carcinoma rates in HBeAg-positive high viral load chronic hepatitis B[J]. J Viral Hepat, 2018, 25(5): 552-560. DOI: 10.1111/jvh.12838. [12] TERRAULT NA, LOK A, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67(4): 1560-1599. DOI: 10.1002/hep.29800. -




 PDF下载 ( 2573 KB)
PDF下载 ( 2573 KB)

 下载:
下载:

 下载:
下载: