直接抗病毒药物改善HCV相关肝细胞癌患者远期预后效果的Meta分析
DOI: 10.3969/j.issn.1001-5256.2021.08.018
利益冲突声明:本研究不存在研究者、伦理委员会成员、受试者监护人以及与公开研究成果有关的利益冲突。
作者贡献声明:周荃负责提出研究选题,设计研究方案,实施研究过程,采集整理数据,起草、修订、终审论文;杨丽晖负责调研整理文献,设计论文框架;蒋芳清负责统计分析,指导性支持。
Long-term prognosis of patients with hepatitis C virus-related hepatocellular carcinoma receiving direct-acting antiviral: A Meta-analysis
-
摘要:
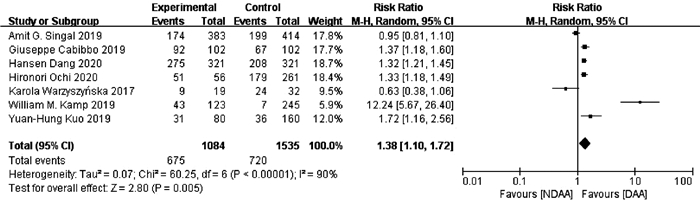
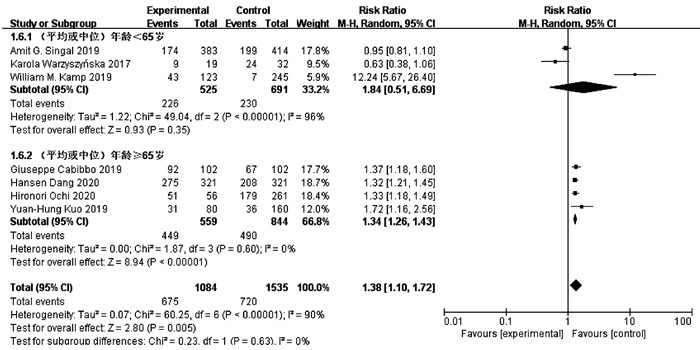
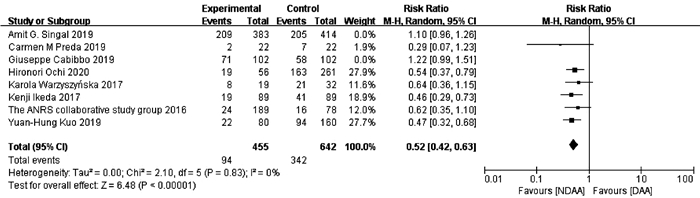
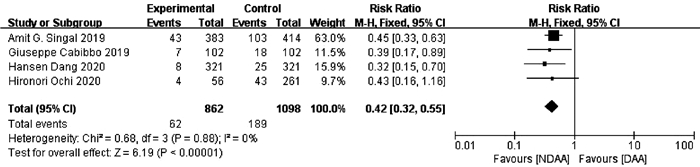
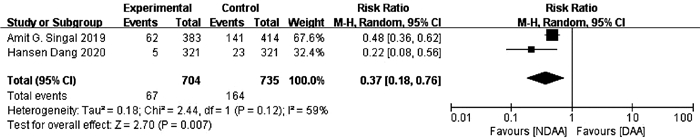
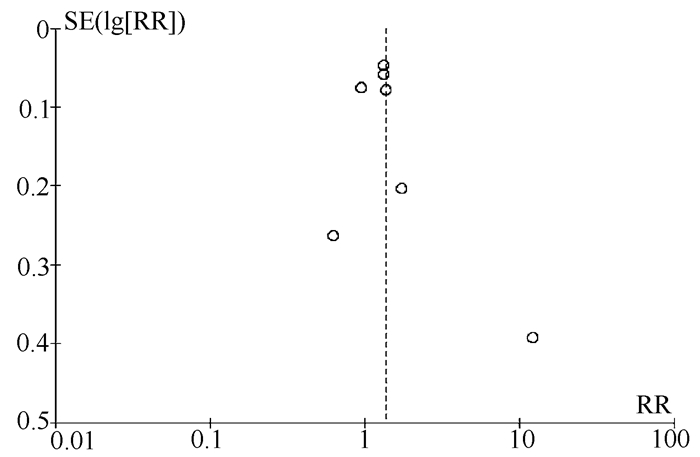
目的 系统的评价全口服直接抗病毒药物(DAA)与无DAA(NDAA)方案对于丙型肝炎相关肝细胞癌(HCC)患者在无复发生存率、HCC复发率、全因死亡率、肝脏相关病死率方面的差异。 方法 检索中国知网、万方数据库、维普数据库、中国生物医学文献数据库、Pubmed、Embase、Cochrane Library七大数据库于2020年12月之前公开发表的DAA治疗丙型肝炎相关HCC患者的临床队列研究,并进行质量评价与Meta分析。 结果 本研究共纳入10个临床队列研究,共计3108例患者。Meta分析结果显示:与NDAA方案相比,DAA治疗能够显著提高丙型肝炎相关HCC患者的无复发生存率(RR=1.38,95%CI:1.10~1.72,P=0.005),降低HCC复发率(RR=0.52,95%CI:0.42~0.63,P<0.000 01)、全因死亡率(RR=0.42,95%CI:0.32~0.55,P<0.000 01)及肝脏相关病死率(RR=0.37,95%CI:0.18~0.76,P=0.007)。 结论 对于丙型肝炎相关HCC患者使用DAA治疗是有益且安全的。 -
关键词:
- 丙型肝炎 /
- 直接抗病毒药物 /
- 癌, 肝细胞 /
- Meta分析(主题)
Abstract:Objective To systematically evaluate the difference in recurrence-free survival rate, hepatocellular carcinoma (HCC) recurrence rate, all-cause mortality rate, and liver-related mortality rate between hepatitis C-related HCC patients receiving oral direct-acting antiviral (DAA) and those receiving non-DAA (NDAA) treatment regimen. Methods CNKI, Wanfang Data, VIP, CBM, PubMed, Embase, and Cochrane Library were searched for Cohort studies of DAA in the treatment of hepatitis C-related HCC patients published before December 2020, and quality assessment and meta-analysis were performed. Results A total of 10 cohort studies were included in this study, with 3108 patients in total. The meta-analysis showed that compared with NDAA regimen, DAA treatment significantly increased recurrence-free survival rate (risk ratio [RR]=1.38, 95% confidence interval [CI]: 1.10-1.72, P=0.005) and significantly reduced HCC recurrence rate (RR=0.52, 95%CI: 0.42-0.63, P < 0.000 01), all-cause mortality rate (RR=0.42, 95%CI: 0.32-0.55, P < 0.000 01), and liver-related mortality rate (RR=0.37, 95%CI: 0.18-0.76, P=0.007) in hepatitis C-related HCC patients. Conclusion DAA treatment is beneficial and safe for hepatitis C-related HCC patients. -
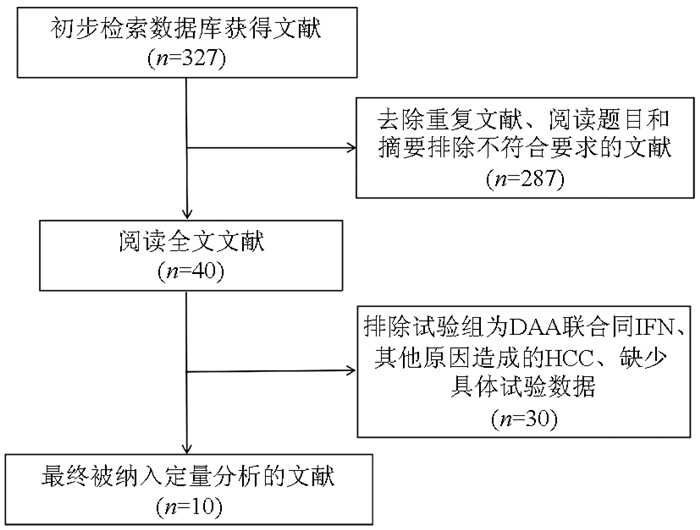
表 1 纳入研究的基本情况
第一作者 年份 总样本量 例数(E/C) 男/女(例) 种族 国籍 年龄(岁) 随访时间 结局指标 NOS评分 Warzyszyńska等[11] 2017 51 19/32 33/18 — 波兰 58.5(36~67) 4年 ①② 8 Singal等[12] 2019 797 383/414 E: 276/107
C: 320/94非西班牙裔白人 美国、加拿大 E: 62.1(58.6~66.1)
C: 61.2(57.0~65.0)4年 ①②③④ 7 Cabibbo等[13] 2019 204 102/102 E: 63/39
C: 65/37— 意大利 E: 71.3±9.5
C: 71.9±8.83年 ①②③ 6 Kamp等[14] 2019 368 123/245 E: 99/24
C: 202/43— — 62.8±10.2 11年 ① 5 Preda等[15] 2019 44 22/22 E: 13/12
C: 9/10— — 64(51~77) 44个月 ② 7 Dang等[16] 2020 642 321/321 E: 197/124
C: 194/127— 美国、日本、韩国、中国台湾 E: 66.39±9.09
C: 65.92±9.135年 ①③④ 6 Kuo等[17] 2019 240 80/160 E: 51/29
C: 102/58— 中国台湾 E: 66
C: 6612年 ①② 6 The ANRS collaborativestudy group[18] 2016 267 189/78 E: 147/42
C: 57/21— 法国 E: 62±9
C: 66±103年 ② 5 Ikeda等[19] 2017 178 89/89 E: 52/37
C: 52/37— 日本 E: 71(39~85)
C: 71(45~85)2年 ② 7 Ochi等[20] 2020 317 56/261 E: 31/25
C: 157/104— 日本 E: 71(56~87)
C: 75(46~91)2年 ①②③ 7 注:E,试验组,C,对照组;①无复发生存率;②HCC复发率;③全因死亡率;④肝脏相关病死率;—,未提及。 -
[1] HOYOS S, ESCOBAR J, CARDONA D, et al. Factors associated with recurrence and survival in liver transplant patients with HCC—a single center retrospective study[J]. Ann Hepatol, 2015, 14(1): 58-63. DOI: 10.1016/S1665-2681(19)30801-4. [2] MCCLUNE AC, TONG MJ. Chronic hepatitis B and hepatocellular carcinoma[J]. Clin Liver Dis, 2010, 14(3): 461-476. DOI: 10.1016/j.cld.2010.05.009. [3] SHI K, ZHANG Q, LI YX, et al. Research advances in the risk prediction models for chronic hepatitis B-related hepatocellular carcinoma[J]. J Clin Hepatol, 2020, 36(10): 2315-2319. DOI: 10.3969/j.issn.1001-5256.2020.10.034.时克, 张群, 李玉鑫, 等. 慢性乙型肝炎相关肝细胞癌风险预测模型的研究进展[J]. 临床肝胆病杂志, 2020, 36(10): 2315-2319. DOI: 10.3969/j.issn.1001-5256.2020.10.034. [4] ZHOU JL, YOU H. Accurate prediction of clinical endpoints of liver cirrhosis[J]. J Clin Hepatol, 2020, 36(9): 1921-1922. DOI: 10.3969/j.issn.1001-5256.2020.09.001.周家玲, 尤红. 肝硬化临床终点事件的精准预测[J]. 临床肝胆病杂志, 2020, 36(9): 1921-1922. DOI: 10.3969/j.issn.1001-5256.2020.09.001. [5] SHEN ZX, LI M, ZHANG JM, et al. Etiologies and clinical features of liver cirrhosis in Lanzhou, China[J]. J Clin Hepatol, 2020, 36(4): 783-787. DOI: 10.3969/j.issn.1001-5256.2020.04.015.沈自雄, 李敏, 张嵴明, 等. 兰州地区肝硬化的病因构成及临床特征分析[J]. 临床肝胆病杂志, 2020, 36(4): 783-787. DOI: 10.3969/j.issn.1001-5256.2020.04.015. [6] KEW MC. Prevention of hepatocellular carcinoma[J]. Ann Hepatol, 2010, 9(2): 120-132. DOI: 10.7326/0003-4819-141-1-200407060-00026. [7] ZEUZEM S. Treatment of chronic hepatitis C virus infection in patients with cirrhosis[J]. J Viral Hepat, 2000, 7(5): 327-334. DOI: 10.1046/j.1365-2893.2000.00229.x. [8] HOU YH, LIU TF, ZHAO XQ, et al. Effect of different direct-acting antivirals on the clinical outcome of genotype 1b chronic hepatitis C and compensated hepatitis C cirrhosis[J]. J Clin Hepatol, 2020, 36(1): 84-87. DOI: 10.3969/j.issn.1001-5256.2020.01.019.侯艺辉, 刘腾飞, 赵晓青, 等. 不同直接抗病毒药物方案对基因1b型慢性丙型肝炎及代偿期丙型肝炎肝硬化临床结局的影响[J]. 临床肝胆病杂志, 2020, 36(1): 84-87. DOI: 10.3969/j.issn.1001-5256.2020.01.019. [9] ZHANG YD, ZHANG YR, WANG H, et al. Clinical features of hepatitis C patients with failure or recurrence after treatment with pegylated interferon-α combined with ribavirin and the clinical effect of direct-acting antiviral agents[J]. J Clin Hepatol, 2019, 35(11): 2456-2460. DOI: 10.3969/j.issn.1001-5256.2019.11.014.张耀弟, 张月荣, 王慧, 等. 聚乙二醇干扰素α联合利巴韦林治疗丙型肝炎失败和复发患者的临床特征及直接抗病毒药物疗效观察[J]. 临床肝胆病杂志, 2019, 35(11): 2456-2460. DOI: 10.3969/j.issn.1001-5256.2019.11.014. [10] ROCHE B, COILLY A, DUCLOS-VALLEE JC, et al. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC[J]. Liver Int, 2018, 38(Suppl 1): 139-145. DOI: 10.1111/liv.13659. [11] WARZYSZYN'SKA K, JONAS M, WASIAK D, et al. Accelerated hepatocellular carcinoma recurrence rate after postoperative direct-acting antivirals treatment - preliminary report[J]. Clin Exp Hepatol, 2017, 3(4): 194-197. DOI: 10.5114/ceh.2017.71483. [12] SINGAL AG, RICH NE, MEHTA N, et al. Direct-acting antiviral therapy for hepatitis C virus infection is associated with increased survival in patients with a history of hepatocellular carcinoma[J]. Gastroenterology, 2019, 157(5): 1253-1263.e2. DOI: 10.1053/j.gastro.2019.07.040. [13] CABIBBO G, CELSA C, CALVARUSO V, et al. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients[J]. J Hepatol, 2019, 71(2): 265-273. DOI: 10.1016/j.jhep.2019.03.027. [14] KAMP WM, SELLERS CM, STEIN S, et al. Impact of direct acting antivirals on survival in patients with chronic hepatitis C and hepatocellular carcinoma[J]. Sci Rep, 2019, 9(1): 17081. DOI: 10.1038/s41598-019-53051-2. [15] PREDA CM, BAICUS C, SANDRA I, et al. Recurrence rate of hepatocellular carcinoma in patients with treated hepatocellular carcinoma and hepatitis C virus-associated cirrhosis after ombitasvir/paritaprevir/ritonavir+dasabuvir+ribavirin therapy[J]. United European Gastroenterol J, 2019, 7(5): 699-708. DOI: 10.1177/2050640619841254. [16] DANG H, YEO YH, YASUDA S, et al. Cure with interferon-free direct-acting antiviral is associated with increased survival in patients with hepatitis C virus-related hepatocellular carcinoma from both East and West[J]. Hepatology, 2020, 71(6): 1910-1922. DOI: 10.1002/hep.30988. [17] KUO YH, WANG JH, CHANG KC, et al. The influence of direct-acting antivirals in hepatitis C virus related hepatocellular carcinoma after curative treatment[J]. Invest New Drugs, 2020, 38(1): 202-210. DOI: 10.1007/s10637-019-00870-9. [18] ANRS collaborative study group on hepatocellular carcinoma. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts[J]. J Hepatol, 2016, 65(4): 734-740. DOI: 10.1016/j.jhep.2016.05.045. [19] IKEDA K, KAWAMURA Y, KOBAYASHI M, et al. Direct-acting antivirals decreased tumor recurrence after initial treatment of hepatitis C virus-related hepatocellular carcinoma[J]. Dig Dis Sci, 2017, 62(10): 2932-2942. DOI: 10.1007/s10620-017-4739-z. [20] OCHI H, HIRAOKA A, HIROOKA M, et al. Direct-acting antivirals improve survival and recurrence rates after treatment of hepatocellular carcinoma within the Milan criteria[J]. J Gastroenterol, 2021, 56(1): 90-100. DOI: 10.1007/s00535-020-01747-y. [21] DASH S, AYDIN Y, WIDMER KE, et al. Hepatocellular carcinoma mechanisms associated with chronic HCV infection and the impact of direct-acting antiviral treatment[J]. J Hepatocell Carcinoma, 2020, 7: 45-76. DOI: 10.2147/JHC.S221187. [22] LI Q, HUANG YX, CHEN L. Research progress in anti-HCV therapeutic regimens without interferon[J]. J Clin Hepatol, 2016, 32(1): 169-173. DOI: 10.3969/j.issn.1001-5256.2016.01.037.李强, 黄玉仙, 陈良. 无干扰素抗HCV方案的研究进展[J]. 临床肝胆病杂志, 2016, 32(1): 169-173. DOI: 10.3969/j.issn.1001-5256.2016.01.037. [23] NAVEED M, ALI A, SHEIKH N, et al. Expression of TRIM22 mRNA in chronic hepatitis C patients treated with direct-acting antiviral drugs[J]. APMIS, 2020, 128(4): 326-334. DOI: 10.1111/apm.13024. [24] XU YW, LI W, CHEN JL. Clinical research on direct antiviral drugs and hepatocellular carcinoma[J/CD]. Chin J Liver Dis(Electronic Edition), 2020, 12(2): 37-41. DOI:10.3969/j.issn.1674-7380.2020.02.007.许雅文, 李威, 陈京龙. 直接抗病毒药物与肝细胞癌临床研究新进展[J/CD]. 中国肝脏病杂志(电子版), 2020, 12(2): 37-41. DOI:10.3969/j.issn.1674-7380.2020.02.007. [25] MARTÍNEZ-CAMPRECIÓS J, BONIS PUIG S, PONS DELGADO M, et al. Transient elastography in DAA era. Relation between post-SVR LSM and histology[J]. J Viral Hepat, 2020, 27(4): 453-455. DOI: 10.1111/jvh.13245. [26] BRUNO S, DI MARCO V, IAVARONE M, et al. Improved survival of patients with hepatocellular carcinoma and compensated hepatitis C virus-related cirrhosis who attained sustained virological response[J]. Liver Int, 2017, 37(10): 1526-1534. DOI: 10.1111/liv.13452. [27] CONTI F, BUONFIGLIOLI F, SCUTERI A, et al. Early occurrence and recurrence of hepatocellular carcinomain HCV-related cirrhosis treated with direct-acting antivirals[J]. J Hepatol, 2016, 65(4) : 727-733. DOI: 10.1016/j.jhep.2020.03.030. [28] REIG M, MARIÑO Z, PERELLÓ C, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy[J]. J Hepatol, 2016, 65(4): 719-726. DOI: 10.1016/j.jhep.2016.04.008. [29] RAVI S, AXLEY P, JONES D, et al. Unusually high rates of hepatocellular carcinoma after treatment with direct-acting antiviral therapy for hepatitis C related cirrhosis[J]. Gastroenterology, 2017, 152(4): 911-912. DOI: 10.1053/j.gastro.2016.12.021. [30] KOSTADINOVA L, SHIVE CL, ZEBROWSKI E, et al. Soluble markers of immune activation differentially normalize and selectively associate with improvement in AST, ALT, albumin, and transient elastography during IFN-free HCV therapy[J]. Pathog Immun, 2018, 3(1): 149-163. DOI: 10.20411/pai.v3i1.242. [31] PERSICO M, ROSATO V, AGLITTI A, et al. Sustained virological response by direct antiviral agents in HCV leads to an early and significant improvement of liver fibrosis[J]. Antivir Ther, 2018, 23(2): 129-138. DOI: 10.3851/IMP3186. [32] DONINI LM, BUSETTO L, BAUER JM, et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review[J]. Clin Nutr, 2020, 39(8): 2368-2388. DOI: 10.1016/j.clnu.2019.11.024. -



 PDF下载 ( 2573 KB)
PDF下载 ( 2573 KB)

 下载:
下载: