葡萄糖-6-磷酸脱氢酶表达与肝细胞癌预后的关系
DOI: 10.3969/j.issn.1001-5256.2021.08.021
Association of the expression of glucose-6-phosphate dehydrogenase with the prognosis of hepatocellular carcinoma
-
摘要:
目的 探究葡萄糖-6-磷酸脱氢酶(G6PD)基因在肝细胞癌(HCC)组织中的表达与患者预后的关系。 方法 收集2016年6月—2018年1月在武汉大学中南医院首诊的44例HCC患者癌组织及对应癌旁组织样本, RT-qRCR检测G6PD mRNA表达, 比较癌组织和癌旁组织G6PD表达水平差异。按照G6PD基因表达水平的中位数将HCC患者分为G6PD高表达组(n=22)和G6PD低表达组(n=22), 结合数据库数据进行分析, 用Kaplan-Meier法绘制生存曲线, 分析两组HCC患者总生存期和无进展生存期的差异。正态分布的计量资料2组间比较采用t检验, 偏态分布的计量资料2组间比较采用Mann-Whitney U检验, 计数资料2组间比较采用Fisher确切概率法; 相关性分析采用Spearman法。 结果 肝癌组织中G6PD mRNA表达水平是癌旁组织的2.09倍, 差异具有统计学意义(Z=-3.221, P=0.001)。G6PD高表达是影响HCC患者肝切除术后总生存期(HR=1.84, 95%CI: 1.30~2.61, P=0.000 52)和无进展生存期(HR=1.75, 95%CI: 1.27~2.42, P=0.000 54)的危险因素。在G6PD低表达组中淋巴细胞/单核细胞比值(LMR)明显高于G6PD高表达组(t=2.681, P=0.011), G6PD与LMR呈负相关(r=-0.439, P=0.005)。 结论 肝癌患者癌组织中G6PD表达升高可能会引起LMR降低, G6PD表达可能与肿瘤微环境炎症有关, G6PD的高表达对HCC的预后评估具有一定的临床价值。 -
关键词:
- 癌, 肝细胞 /
- 葡萄糖-6-磷酸脱氢酶 /
- 预后
Abstract:Objective To investigate the expression of glucose-6-phosphate dehydrogenase (G6PD) in hepatocellular carcinoma (HCC) tissue and its association with prognosis. Methods HCC tissue samples and corresponding adjacent tissue samples were collected from 44 HCC patients who attended Zhongnan Hospital of Wuhan University from June 2016 to January 2018. RT-qRCR was used to measure the mRNA expression of G6PD, and the expression level of G6PD was compared between HCC tissue and adjacent tissue. According to the median of the mRNA expression of G6PD, HCC patients were divided into high G6PD expression group and low G6PD expression group and were analyzed with reference to the data in databases, and the Kaplan-Meier method was used to plot survival curves and investigate the differences in overall survival time and progression-free survival time between the two groups. The t-test was used for comparison of normally distributed continuous data between the two groups, and the Mann-Whitney U test was used for comparison of data with skewed distribution between the two groups; the Fisher's exact test was used for comparison of categorical data between the two groups; the Spearman method was used to perform a correlation analysis. Results The mRNA expression level of G6PD in HCC tissue was 2.09 times that in adjacent tissue (Z=-3.221, P=0.001). High expression of G6PD was a risk factor for overall survival time (hazard ratio [HR]=1.84, 95% confidence interval [CI: 1.30-2.61, P=0.000 52) and progression-free survival time (HR=1.75, 95%CI: 1.27-2.42, P=0.000 54) in HCC patients after hepatectomy. The low G6PD expression group had a significantly higher lymphocyte-monocyte ratio (LMR) than the high G6PD expression group (t=2.681, P=0.011), and G6PD was negatively correlated with LMR (r=-0.439, P=0.005). Conclusion Elevated expression of G6PD in HCC tissue may lead to a reduction in LMR in HCC patients, suggesting that G6PD expression might be associated with inflammation in tumor microenvironment. High expression of G6PD has a certain clinical value in evaluating the prognosis of HCC. -
Key words:
- Carcinoma, Hepatocellular /
- G6PD /
- Prognosis
-
肝细胞癌(HCC)是常见的消化系统恶性肿瘤之一, 其恶性程度高, 增殖能力强, 患者预后差。2018年, 全球新发肝癌84.1万例, 在恶性肿瘤发病率排名第6位, 死亡78.1万例[1]; 2015年我国的新发肝癌约37.0万例, 死亡人数约32.6万例[2]。因此, 寻找肝癌预后评估的生物标志物具有重要的临床意义。
葡萄糖-6-磷酸脱氢酶(glucose-6-phosphate dehydrogenase, G6PD是参与磷酸戊糖途径(pentose phosphate pathway, PPP)的第一种酶, 也是PPP的限速酶, 糖酵解产生的6-磷酸葡萄糖在G6PD的催化下进入PPP, 产生细胞活动所需要的能量[3]。研究发现, G6PD在黑色素瘤[4]、乳腺癌[5]、肺癌[6-7]、肝癌[8-9]、结直肠癌[10]等恶性肿瘤中表达升高, 在肿瘤的发生发展中起重要作用。
淋巴细胞/单核细胞比值(LMR)是反映机体炎症和免疫状态的一项敏感指标, 已被证实可用于预测卵巢癌[11]、肺癌[12]和肝癌[13]等实体肿瘤的预后。本文通过分析G6PD在肝癌组织中的表达与预后的关系, 以及不同G6PD表达水平患者的临床指标差异, 探讨G6PD表达在HCC预后评估中的临床价值。
1. 资料与方法
1.1 研究对象
收集2016年6月—2018年1月在本院就诊的44例首诊HCC患者的肝癌组织和相应的癌旁组织标本, 及其临床信息和实验室检查结果。纳入标准: (1)所有患者均符合《原发性肝癌诊疗规范(2019年版)》[14]; (2)有完整的基本资料和实验室检测结果(肝肾功能、血常规、凝血指标和肿瘤标志物)。排除标准: (1)合并其他恶性肿瘤或有全身感染症状; (2)术前接受过其他方式治疗。
1.2 仪器试剂
采用贝克曼奥林巴斯5800全自动生化分析仪检测肝肾功能, 贝克曼库尔特全自动血细胞分析仪H750检测血常规。贝克曼ACLTOP检测凝血指标。BeckmanDX1800全自动化学发光仪测定AFP。BIO-RAD荧光定量PCR系统CFX ConnectTM检测基因表达。
1.3 方法
使用Trizol(美国Thermo公司)提取组织RNA。逆转录试剂盒FSQ-301(日本TOYOBO公司)合成cDNA。β-actin为内参基因, 进行qPCR。G6PD: 上游5′-AACATCGCCTGCGTTATCCTC-3′, 下游5′-ACGTCCCGGATGATCCCAA-3′; β-actin: 上游5′-CCTCGCCTTTGCCGATCC-3′, 下游5′-GGATCTTCATGAGGTAGTC AGTC-3′。
用Gene Expression Profiling Interactive Analysis(GEPIA)数据库分析G6PD在HCC组织中的表达。Kaplan Meier Plotter(KM plotter)数据库评估HCC组织中G6PD表达水平与预后。
1.4 伦理学审查
本研究方案经由武汉大学中南医院伦理委员会审批, 批号: 2017058。
1.5 统计学方法
采用SPSS 21.0软件进行数据分析。正态分布的计量资料以x±s表示, 2组间比较采用t检验; 偏态分布的计量资料以M(P25~P75)表示, 2组间比较采用Mann-Whitney U检验。计数资料2组间比较采用Fisher确切概率法; Spearman相关用来分析G6PD与LMR的相关性; 采用Kaplan-Meier法绘制生存曲线。P<0.05为差异有统计学意义。
2. 结果
2.1 肝癌组织中G6PD mRNA表达
运用RT-qPCR检测44例HCC患者肝癌组织和癌旁肝脏组织中G6PD的mRNA表达水平。结果发现, G6PD mRNA在癌组织中表达明显高于癌旁组织(Z=-3.221, P=0.001), 癌组织G6PD表达水平是癌旁组织的2.09倍(图 1a)。
2.2 低G6PD组和高G6PD组的HCC患者基本资料
根据G6PD mRNA表达水平中位数, 将患者分为G6PD高表达组(n=22)及低表达组(n=22)。两组患者在年龄、性别、HBV DNA检测阳性率和TNM分期上差异均无统计学意义(P值均>0.05)(表 1)。
表 1 HCC患者低G6PD组和高G6PD组的基本资料指标 低G6PD组(n=22) 高G6PD组(n=22) P值 年龄(岁) 57.04±9.88 52.00±8.74 0.080 男/女(例) 17/5 17/5 1.000 HBV DNA(例) 0.537 <500 IU/ml 7 10 ≥500 IU/ml 15 12 TNM分期(例) 0.736 Ⅰ~Ⅱ 15 17 Ⅲ~Ⅳ 7 5 2.3 肝癌患者预后与G6PD表达的关系分析
运用GEPIA数据库分析肝癌组织(n=369)和癌旁组织(n=160)中的G6PD表达。结果发现, G6PD在癌组织中表达明显高于癌旁组织(P<0.01)(图 1b)。运用KM plotter数据库, 分析两组患者预后差异。结果发现, G6PD高表达是不良预后的危险因素: 总生存期(HR=1.84, 95%CI: 1.30~2.61; P=0.000 52)和无进展生存期(HR=1.75, 95%CI: 1.27~2.42;P=0.000 54), 即G6PD高表达患者预后不良(图 1c、d)。
2.4 肝癌组织中G6PD表达与临床指标的关系
为了进一步比较肝癌患者中G6PD mRNA表达与临床指标的关系, 比较HCC患者低G6PD组(n=22)和高G6PD组(n=22)的肝功能、血常规、凝血功能和AFP差异。
结果显示, LMR在高G6PD组患者中明显低于低G6PD组(P=0.011), 其他指标两组间差异均无统计学意义(P值均>0.05)(表 2)。
表 2 肝癌患者G6PD表达水平与术前临床指标关系指标 低G6PD组(n=22) 高G6PD组(n=22) 统计值 P值 ALT(U/L) 41.09±28.89 43.64±39.56 t=-0.244 0.809 AST(U/L) 49.00±33.74 47.23±30.33 t=0.183 0.855 总胆红素(μmol/L) 16.60±6.83 20.44±8.93 t=-1.604 0.116 直接胆红素(μmol/L) 4.49±2.82 4.94±2.20 t=-0.579 0.566 间接胆红素(μmol/L) 12.35±4.96 15.05±7.87 t=-1.335 0.187 TP(g/L) 66.80±5.96 67.58±6.27 t=-0.422 0.675 Alb(g/L) 39.66±3.75 39.65±5.63 t=0.006 0.995 Glb(g/L) 27.14±4.17 27.92±4.82 t=-0.575 0.568 GGT(U/L) 61.00±46.68 66.05±43.69 t=-0.366 0.716 ALP(U/L) 103.82±52.41 108.05±56.92 t=-0.256 0.799 WBC(×109/L) 5.35±1.66 5.61±2.34 t=-0.413 0.682 RBC(×1012/L) 4.24±0.85 4.41±0.70 t=-0.691 0.494 HGB(g/L) 129.59±25.11 135.34±18.16 t=-0.844 0.404 PLT(×109/L) 150.10±54.03 173.19±82.75 t=-1.052 0.229 中性粒细胞计数(×109/L) 3.41±1.64 3.80±1.80 t=-0.717 0.478 淋巴细胞计数(×109/L) 1.39±0.55 1.19±0.59 t=1.116 0.271 单核细胞计数(×109/L) 0.42±0.14 0.50±0.21 t=-1.531 0.134 LMR 3.49±1.44 2.45±0.96 t=2.681 0.011 PT(s) 11.59±1.06 11.70±0.88 t=-0.348 0.730 INR 1.06±0.10 1.07±0.08 t=-0.295 0.770 PTTA(%) 97.81±14.29 97.15±15.26 t=0.143 0.887 APTT(s) 32.20±2.78 32.63±3.65 t=-0.433 0.668 TT(s) 14.97±1.22 14.30±1.77 t=1.438 0.158 FIB(mg/dl) 286.61±56.74 308.19±81.70 t=-0.994 0.326 AFP(μg/L) 143.80(6.22~2 386.96) 1 481.35(56.20~4 453.63) Z=-1.723 0.085 2.5 G6PD表达与LMR相关性分析
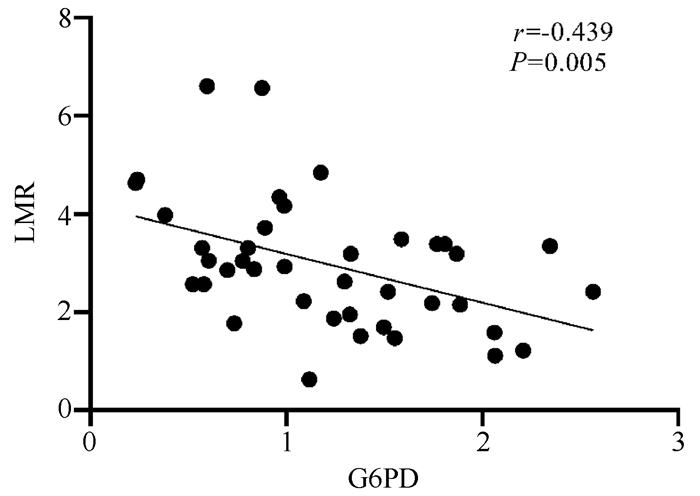
进一步分析G6PD mRNA表达量与LMR相关性, 结果显示G6PD表达水平与LMR呈负相关(r=-0.439, P=0.005)(图 2), 提示G6PD表达与免疫功能、炎症状态有关。
3. 讨论
HCC是一种进展迅速, 恶性程度高, 预后极差的肿瘤[15]。随着各种分子生物学层面的研究深入, 越来越多的研究表明肝癌的发生发展常伴随着糖代谢紊乱[16]、多种基因表达改变和信号通路异常[17]。
肿瘤细胞由于瓦伯格效应(Warburg effect)主要通过糖酵解的方式分解葡萄糖获能, 所产生的6-磷酸葡萄糖可以通过PPP为核苷酸、脂质等生物分子合成提供前体以及NADPH[16, 18]。本研究发现G6PD基因在HCC中表达明显高于配对癌旁组织。相关研究指出, PI3K/Akt、Ras和Src等促癌信号通路的过度激活, 可通过翻译后调控机制促进G6PD激活[3], 从而引起肿瘤组织中G6PD表达升高, 产生的高水平NADPH可以减少细胞活性氧(ROS)的生成[19]。ROS在多种癌症中被检测到, 一方面被认为可以激活肿瘤信号, 但同时ROS累积也能启动氧化应激诱导肿瘤细胞的死亡, 肿瘤细胞G6PD表达升高, 可以减少升高的ROS, 建立氧化还原平衡[20]。同时细胞内ROS累积会引起NF-κB的激活, 可引起大量细胞因子如IL-6、IL-24、IL-32等的释放, 细胞因子招募单核细胞、巨噬细胞到肿瘤部位, 引起肿瘤微环境炎症反应, 促进肿瘤发展[21]。
已经有研究证实HBV感染会引起G6PD的升高[22], 同时HBV感染可能会引起肿瘤微环境炎症反应, 引起免疫细胞数量和状态改变[23]。而高LMR是多种癌症预后的保护因素[9, 24], 在肝癌的相关研究[25]中, 肝癌术前LMR可分为高值组(≥3.03)和低值组(<3.03), 高LMR组患者肝癌切除术后5年生存率较好。也有学者[26]指出肝移植术后LMR<2.75的患者的5年生存率明显低于LMR≥2.75的患者。而本研究发现LMR在G6PD高表达组中明显低于G6PD低表达组, 且肝癌组织中G6PD表达水平与LMR呈负相关, 提示G6PD高表达的HCC患者不良预后可能与肿瘤微环境炎症有关。
综上, 在肝癌组织中高G6PD的表达与不良预后有关, G6PD高表达和低表达组的LMR有明显差异, 且G6PD的表达量与LMR呈负相关, 提示G6PD表达可能与肿瘤微环境炎症反应有关。本研究所用样本量较小, 具体的分子机制尚需进一步研究。
-
表 1 HCC患者低G6PD组和高G6PD组的基本资料
指标 低G6PD组(n=22) 高G6PD组(n=22) P值 年龄(岁) 57.04±9.88 52.00±8.74 0.080 男/女(例) 17/5 17/5 1.000 HBV DNA(例) 0.537 <500 IU/ml 7 10 ≥500 IU/ml 15 12 TNM分期(例) 0.736 Ⅰ~Ⅱ 15 17 Ⅲ~Ⅳ 7 5 表 2 肝癌患者G6PD表达水平与术前临床指标关系
指标 低G6PD组(n=22) 高G6PD组(n=22) 统计值 P值 ALT(U/L) 41.09±28.89 43.64±39.56 t=-0.244 0.809 AST(U/L) 49.00±33.74 47.23±30.33 t=0.183 0.855 总胆红素(μmol/L) 16.60±6.83 20.44±8.93 t=-1.604 0.116 直接胆红素(μmol/L) 4.49±2.82 4.94±2.20 t=-0.579 0.566 间接胆红素(μmol/L) 12.35±4.96 15.05±7.87 t=-1.335 0.187 TP(g/L) 66.80±5.96 67.58±6.27 t=-0.422 0.675 Alb(g/L) 39.66±3.75 39.65±5.63 t=0.006 0.995 Glb(g/L) 27.14±4.17 27.92±4.82 t=-0.575 0.568 GGT(U/L) 61.00±46.68 66.05±43.69 t=-0.366 0.716 ALP(U/L) 103.82±52.41 108.05±56.92 t=-0.256 0.799 WBC(×109/L) 5.35±1.66 5.61±2.34 t=-0.413 0.682 RBC(×1012/L) 4.24±0.85 4.41±0.70 t=-0.691 0.494 HGB(g/L) 129.59±25.11 135.34±18.16 t=-0.844 0.404 PLT(×109/L) 150.10±54.03 173.19±82.75 t=-1.052 0.229 中性粒细胞计数(×109/L) 3.41±1.64 3.80±1.80 t=-0.717 0.478 淋巴细胞计数(×109/L) 1.39±0.55 1.19±0.59 t=1.116 0.271 单核细胞计数(×109/L) 0.42±0.14 0.50±0.21 t=-1.531 0.134 LMR 3.49±1.44 2.45±0.96 t=2.681 0.011 PT(s) 11.59±1.06 11.70±0.88 t=-0.348 0.730 INR 1.06±0.10 1.07±0.08 t=-0.295 0.770 PTTA(%) 97.81±14.29 97.15±15.26 t=0.143 0.887 APTT(s) 32.20±2.78 32.63±3.65 t=-0.433 0.668 TT(s) 14.97±1.22 14.30±1.77 t=1.438 0.158 FIB(mg/dl) 286.61±56.74 308.19±81.70 t=-0.994 0.326 AFP(μg/L) 143.80(6.22~2 386.96) 1 481.35(56.20~4 453.63) Z=-1.723 0.085 -
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. [2] AN L, ZENG HM, ZHENG RS, et al. Liver cancer epidemiology in china, 2015[J]. Chin J Oncol, 2019, 41(10): 721-727. DOI: 10.3760/cma.j.issn.0253?3766.2019.10.001.安澜, 曾红梅, 郑荣寿, 等. 2015年中国肝癌流行情况分析[J]. 中华肿瘤杂志, 2019, 41(10): 721-727. DOI: 10.3760/cma.j.issn.0253?3766.2019.10.001. [3] PATRA KC, HAY N. The pentose phosphate pathway and cancer[J]. Trends Biochem Sci, 2014, 39(8): 347-354. DOI: 10.1016/j.tibs.2014.06.005. [4] CAI T, KUANG Y, ZHANG C, et al. Glucose-6-phosphate dehydrogenase and NADPH oxidase 4 control STAT3 activity in melanoma cells through a pathway involving reactive oxygen species, c-SRC and SHP2[J]. Am J Cancer Res, 2015, 5(5): 1610-1620. http://europepmc.org/articles/PMC4497430 [5] MELE L, LA NOCE M, PAINO F, et al. Glucose-6-phosphate dehydrogenase blockade potentiates tyrosine kinase inhibitor effect on breast cancer cells through autophagy perturbation[J]. J Exp Clin Cancer Res, 2019, 38(1): 160. DOI: 10.1186/s13046-019-1164-5. [6] ZHANG R, TAO F, RUAN S, et al. The TGFβ1-FOXM1-HMGA1-TGFβ1 positive feedback loop increases the cisplatin resistance of non-small cell lung cancer by inducing G6PD expression[J]. Am J Transl Res, 2019, 11(11): 6860-6876. http://www.ncbi.nlm.nih.gov/pubmed/31814893 [7] RAO X, DUAN X, MAO W, et al. O-GlcNAcylation of G6PD promotes the pentose phosphate pathway and tumor growth[J]. Nat Commun, 2015, 6: 8468. DOI: 10.1038/ncomms9468. [8] BARAJAS JM, REYES R, GUERRERO MJ, et al. The role of miR-122 in the dysregulation of glucose-6-phosphate dehydrogenase (G6PD) expression in hepatocellular cancer[J]. Sci Rep, 2018, 8(1): 9105. DOI: 10.1038/s41598-018-27358-5. [9] PES GM, ERRIGO A, SORO S, et al. Glucose-6-phosphate dehydrogenase deficiency reduces susceptibility to cancer of endodermal origin[J]. Acta Oncol, 2019, 58(9): 1205-1211. DOI: 10.1080/0284186X.2019.1616815. [10] ZHANG X, ZHANG X, LI Y, et al. PAK4 regulates G6PD activity by p53 degradation involving colon cancer cell growth[J]. Cell Death Dis, 2017, 8(5): e2820. DOI: 10.1038/cddis.2017.85. [11] TANG Y, HU HQ, TANG FX, et al. Combined preoperative LMR and CA125 for prognostic assessment of ovarian cancer[J]. J Cancer, 2020, 11(11): 3165-3171. DOI: 10.7150/jca.42477. [12] MANDALIYA H, JONES M, OLDMEADOW C, et al. Prognostic biomarkers in stage Ⅳ non-small cell lung cancer (NSCLC): Neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI)[J]. Transl Lung Cancer Res, 2019, 8(6): 886-894. DOI: 10.21037/tlcr.2019.11.16. [13] ISMAEL MN, FORDE J, MILLA E, et al. Utility of inflammatory markers in predicting hepatocellular carcinoma survival after liver transplantation[J]. Biomed Res Int, 2019, 2019: 7284040. DOI: 10.1155/2019/7284040. [14] Bureau of Medical Administration, National Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007.中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗规范(2019年版)[J]. 临床肝胆病杂志, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. [15] European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma[J]. J Hepatol, 2018, 69(1): 182-236. DOI: 10.1016/j.jhep.2018.03.019. [16] SHANG RZ, QU SB, WANG DS. Reprogramming of glucose metabolism in hepatocellular carcinoma: Progress and prospects[J]. World J Gastroenterol, 2016, 22(45): 9933-9943. DOI: 10.3748/wjg.v22.i45.9933. [17] ZUCMAN-ROSSI J, VILLANUEVA A, NAULT JC, et al. Genetic landscape and biomarkers of hepatocellular carcinoma[J]. Gastroenterology, 2015, 149(5): 1226-1239. e4. DOI: 10.1053/j.gastro.2015.05.061. [18] LU M, LU L, DONG Q, et al. Elevated G6PD expression contributes to migration and invasion of hepatocellular carcinoma cells by inducing epithelial-mesenchymal transition[J]. Acta Biochim Biophys Sin (Shanghai), 2018, 50(4): 370-380. DOI: 10.1093/abbs/gmy009. [19] SOSA V, MOLINÉ T, SOMOZA R, et al. Oxidative stress and cancer: An overview[J]. Ageing Res Rev, 2013, 12(1): 376-390. DOI: 10.1016/j.arr.2012.10.004. [20] MOLONEY JN, COTTER TG. ROS signalling in the biology of cancer[J]. Semin Cell Dev Biol, 2018, 80: 50-64. DOI: 10.1016/j.semcdb.2017.05.023. [21] YAN YC, WEN K, MAO K, et al. Pathogenesis of hepatitis B virus-related hepatocellular carcinoma[J]. J Clin Hepatol, 2020, 36(10): 2167-2172. DOI: 10.3969/j.issn.1001-5256.2020.10.002.颜永聪, 温凯, 毛凯, 等. HBV相关肝细胞癌的发生机制[J]. 临床肝胆病杂志, 2020, 36(10): 2167-2172. DOI: 10.3969/j.issn.1001-5256.2020.10.002. [22] LIU B, FANG M, HE Z, et al. Hepatitis B virus stimulates G6PD expression through HBx-mediated Nrf2 activation[J]. Cell Death Dis, 2015, 6: e1980. DOI: 10.1038/cddis.2015.322. [23] LIM CJ, LEE YH, PAN L, et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma[J]. Gut, 2019, 68(5): 916-927. DOI: 10.1136/gutjnl-2018-316510. [24] CHEN XQ, XUE CR, HOU P, et al. Lymphocyte-to-monocyte ratio effectively predicts survival outcome of patients with obstructive colorectal cancer[J]. World J Gastroenterol, 2019, 25(33): 4970-4984. DOI: 10.3748/wjg.v25.i33.4970. [25] ZHU YW, YAO MJ, BO YC, et al. Prognostic assessment of lymphocyte-to-monocyte ratio for patients with primary liver cancer after curative hepatectomy[J]. J Zhengzhou Univ (Medical Sciences), 2017, 52(2): 197-200. DOI: 10.13705/j.issn.1671-6825.2017.02.023.朱艺伟, 姚明解, 薄亚聪, 等. 淋巴细胞与单核细胞比值对原发性肝癌患者根治性切除术后预后的预测价值[J]. 郑州大学学报(医学版), 2017, 52(2): 197-200. DOI: 10.13705/j.issn.1671-6825.2017.02.023. [26] MANO Y, YOSHIZUMI T, YUGAWA K, et al. Lymphocyte-to-monocyte ratio is a predictor of survival after liver transplantation for hepatocellular carcinoma[J]. Liver Transpl, 2018, 24(11): 1603-1611. DOI: 10.1002/lt.25204. 期刊类型引用(1)
1. 姚元谦,吕建林,柳琳琳,王光耀. 铁死亡基因及相关长链非编码RNA在肝细胞癌发生与预后中的作用. 中国医药导报. 2022(26): 9-14 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 2593 KB)
PDF下载 ( 2593 KB)


 下载:
下载:


 下载:
下载:
 百度学术
百度学术



