腹腔镜肝切除术难度评分系统的临床应用价值
DOI: 10.3969/j.issn.1001-5256.2021.08.027
Clinical application value of difficulty score systems before laparoscopic liver resection
-
摘要:
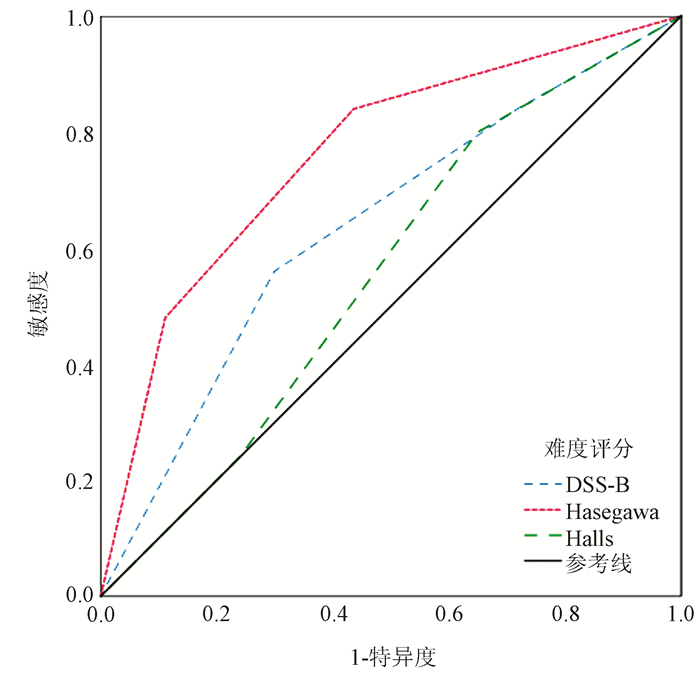
目的 探讨3种腹腔镜肝切除术难度评分系统(DSS)评估手术难度和预测短期术后结果的准确性。 方法 选取2015年6月—2020年5月于兰州大学第一医院行腹腔镜肝切除术的患者142例,收集患者术前、术中和术后临床资料。根据术前资料,用DSS-B评分、Hasegawa评分和Halls评分3种DSS对每个患者手术的难度进行评分并分为低、中、高难度3组,比较各组间术中资料的差异,以验证3种DSS的准确性;用术后资料评估DSS对短期术后结果的预测能力。正态分布的计量资料多组间比较采用方差分析,进一步两两比较采用LSD-t检验;非正态分布的计量资料多组间比较及进一步两两比较均采用Kruskal-Wallis H检验。计数资料组间比较采用χ2检验或Fisher精确概率法,两两比较的P值用Bonferroni法校正。通过绘制受试者工作特征曲线(ROC曲线)并计算曲线下面积(AUC),评价DSS对术后并发症的预测效能。 结果 142例患者中,DSS-B评分低、中、高难度组分别有37、56、49例;Hasegawa评分低、中、高难度组分别有70、47、25例;Halls评分低、中、高难度组分别有46、62、34例。DSS-B评分、Hasegawa评分和Halls评分在低、中、高难度组之间手术时间、术中出血量、肝门阻断率均随难度评分的升高而增高(P值均<0.001);术中输血率方面,DSS-B评分仅在中难度与高难度组间差异有统计学意义(P<0.017),Halls评分在低难度与高难度组间差异有统计学意义(P<0.017),而Hasegawa评分低难度与中难度、高难度组比较,差异均有统计学意义(P值均<0.017);中转开腹率方面,DSS-B评分中难度与高难度组间差异有统计学意义(P<0.017),Hasegawa评分、Halls评分可区分低难度与高难度组间差异(P值均<0.017)。术后住院时间方面DSS-B评分和Halls评分均只能区分低难度与高难度组间差异(P值均<0.05),Hasegawa评分则可区分低难度与中难度、高难度的组间差异(P值均<0.05);术后并发症发生率方面仅Hasegawa评分可有效区分高难度与低难度、中难度的组间差异(P值均<0.017)。DSS-B评分、Halls评分和Hasegawa评分预测术后并发症的AUC分别为0.636(95%CI:0.515~0.758)、0.557(95%CI:0.442~0.673)、0.760(95%CI:0.654~0.866),其中Hasegawa评分的预测效能最大。 结论 DSS-B评分和Hasegawa评分可较好地评估腹腔镜肝切除术难度,Hasegawa评分在预测短期术后结果方面具有优势。 Abstract:Objective To investigate the accuracy of three laparoscopic liver resection (LLR) difficulty score systems (DSSs) in evaluating surgical difficulty and predicting short-term postoperative outcome. Methods The retrospective cohort study was conducted for 142 patients who underwent LLR in The First Hospital of Lanzhou University from June 2015 to May 2020, and their preoperative, intraoperative, and postoperative clinical data were collected. According to preoperative clinical data, DSS-B score, Hasegawa score, and Halls score were used to determine the difficulty score of surgery for each patient, and then the patients were divided into low, medium, and high difficulty groups. Intraoperative data were compared between the three groups to verify the accuracy of the three DSSs, and postoperative clinical data were used to evaluate the ability of DSSs to predict short-term postoperative outcome. An analysis of variance was used for comparison of normally distributed continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups; the Kruskal-Wallis H test was used for comparison of non-normally distributed continuous data between multiple or two groups. The chi-square test or the Fisher's exact test was used for comparison of categorical data between groups, and the Bonferroni method was used for correction of P values between two groups. The receiver operating characteristic (ROC) curve was plotted and the area under the ROC curve (AUC) was calculated to evaluate the efficiency of each DSS in predicting postoperative complications. Results Among the 142 patients, there were 37 patients in the low difficulty group, 56 in the medium difficulty group, and 49 in the high difficulty group based on DSS-B score; there were 70 patients in the low difficulty group, 47 in the medium difficulty group, and 25 in the high difficulty group based on Hasegawa score; there were 46 patients in the low difficulty group, 62 in the medium difficulty group, and 34 in the high difficulty group based on Halls score. For the low, medium, and high difficulty groups based on DSS-B score, Hasegawa score, or Halls score, time of operation, intraoperative blood loss, and rate of hepatic portal occlusion increased with the increase in difficulty score (all P < 0.001); there was a significant difference in intraoperative blood transfusion rate between the medium and high difficulty groups based on DSS-B score (P < 0.017), between the low and high difficulty groups based on Halls score (P < 0.017), and between the low, medium, and high difficulty groups based on Hasegawa score (P < 0.017). There was a significant difference in the rate of conversion to laparotomy between the medium and high difficulty groups based on DSS-B score (P < 0.017), and Hasegawa score and Halls score identified the difference between the low and high difficulty groups (P < 0.017). For the length of postoperative hospital stay, DSS-B score and Halls score only identified the difference between the low and high difficulty groups (P < 0.05), while Hasegawa score identified the difference between the low difficulty group and the medium/high difficulty groups (P < 0.05); for the incidence rate of postoperative complications, only Hasegawa score effectively identified the difference between the high difficulty group and the low/medium difficulty groups (P < 0.017). DSS-B score, Halls score, and Hasegawa score had an AUC of 0.636 (95% confidence interval [CI]: 0.515-0.758), 0.557 (95% CI: 0.442-0.673), and 0.760 (95% CI: 0.654-0.866), respectively, in predicting postoperative complications, among which Hasegawa score had the highest predictive efficiency. Conclusion DSS-B score and Hasegawa score can better assess the difficulty of LLR, and Hasegawa score has an advantage in predicting short-term postoperative outcome. -
Key words:
- Hiver Diseases /
- Laparoscopy /
- Hepatectomy /
- Difficulty Scores System
-
表 1 3种腹腔镜肝切除难度评分表
评分类型 评分标准 难度等级 DSS-B评分 ①肿瘤位置(Ⅲ:1分;Ⅱ、Ⅵ:2分;Ⅳ、Ⅴ:3分;Ⅶ、Ⅷ:5分) 低:1~3分 ②肿瘤大小(<3 cm:0分;≥3 cm:1分) 中:4~6分 ③肝切除范围(部分楔形切除:0分;左外叶切除:2分;肝段切除:3分;肝叶切除:4分) 高:7~10分 ④肿瘤接近主要脉管(无:0分;有:1分) ⑤肝功能Child-Pugh分级(A级:0分;B级:1分) Hasegawa评分 ①肿瘤位置(Ⅱ~Ⅳ:0分;Ⅴ、Ⅵ:1分;Ⅶ、Ⅷ:2分) 低:0~1分 ②肝切除范围(非解剖肝切除或腹腔镜下肝左外叶切除:0分;解剖性肝段切除:2分;肝大部切除:3分) 中:2~3分 ③PLT(>100×109/L:0分;≤100×109/L:1分) 高:≥4分 ④BMI(<30 kg/m2:0分;≥30 kg/m2:1分) Halls评分 ①病灶大小(<3 cm:0分;3~5 cm:2分;>5 cm:3分) 低:0~2分 ②肿物性质(良性:0分;恶性:2分) 中:3~5分 ③肝切除类型(小范围切除:0分;技术性大切除:2分;解剖性大部切除:4分) 高:6~9分 ④既往开腹肝脏手术史(无:0分;有:5分) 极高:≥10分 ⑤术前化疗(无:0分;有:1分) 注:Ⅰ~Ⅷ,肝Couinaud分段。 表 2 患者临床基线资料
项目 数值 男/女(例) 86/56 年龄(岁) 51.56±11.54 BMI(kg/m2) 23.25±3.34 麻醉ASA分级(Ⅰ/Ⅱ/Ⅲ,例) 2/111/29 肝硬化[例(%)] 76(53.5) 肝功能Child-Pugh分级(A/B/C,例) 132/10/0 诊断(例) 肝细胞癌 86 肝内胆管癌 8 肝血管瘤 22 肝包虫 16 肝脏局灶性结节增生 7 其他 3 既往开腹肝脏手术史[例(%)] 6(4.2) 术前化疗史[例(%)] 7(4.9) 中转开腹[例(%)] 10(7.0) 肝门阻断[例(%)] 74(52.1) 术中输血[例(%)] 13(9.2) 术后并发症(例) Ⅰ级 16 Ⅱ级 5 Ⅲa级 1 Ⅲb级 2 Ⅳa级 1 术后主要并发症[例(%)] 4(2.8) 难度分级 DSS-B评分(低/中/高,例) 37/56/49 Hasegawa评分(低/中/高,例) 70/47/25 Halls评分(低/中/高/极高,例) 46/62/34/0 表 3 3种DSS各难度组间比较
组别 例数 术中指标 术后指标 手术时间(min) 术中出血量(ml) 肝门阻断率[例(%)] 术中输血率[例(%)] 中转开腹率[例(%)] 术后住院时间(d) 术后并发症发生率[例(%)] DSS-B评分 低难度组 37 150.5±73.9 90(20~190) 6(16.2) 2(5.4) 1(2.7) 6(4~8) 4(10.8) 中难度组 56 242.6±80.51) 200(100~300)1) 23(41.1)1) 1(1.8) 1(1.8) 7(6~9) 7(12.5) 高难度组 49 364.7±112.61)2) 300(200~550)1)2) 45(91.8)1)2) 10(20.4)2) 8(16.3)2) 8(7~10)1) 14(28.6) 统计值 F=59.651 H=38.847 χ2=52.825 χ2=10.628 χ2=8.337 H=14.723 χ2=6.246 P值 <0.001 <0.001 <0.001 0.004 0.013 0.001 0.045 Hasegawa评分 低难度组 70 191.4±83.2 100(50~200) 20(28.6) 1(1.4) 2(2.9) 6(5~8) 4(5.7) 中难度组 47 289.8±100.61) 200(100~400)1) 29(61.7)1) 6(12.8)1) 3(6.4) 8(7~10)1) 9(19.1) 高难度组 25 400.4±121.21)2) 500(250~600)1)2) 25(100.0)1)2) 6(24.0)1) 5(20.0)1) 8(7~12)1) 12(48.0)1)2) 统计值 F=46.383 H=41.574 χ2=40.250 χ2=12.507 χ2=6.947 H=21.164 χ2=20.665 P值 <0.001 <0.001 <0.001 0.001 0.022 <0.001 <0.001 Halls评分 低难度组 46 173.9±86.4 100(45~200) 8(17.4) 0 1(2.2) 7(4~9) 5(10.9) 中难度组 62 273.9±97.51) 200(100~325)1) 36(58.1)1) 7(11.3) 2(3.2) 7(6~9) 14(22.6) 高难度组 34 354.1±133.51)2) 300(200~600)1) 30(88.2)1)2) 6(17.6)1) 7(20.6)1) 8(7~10)1) 6(17.6) 统计值 F=30.201 H=33.263 χ2=40.880 χ2=9.136 χ2=9.766 H=11.978 χ2=2.497 P值 <0.001 <0.001 <0.001 0.008 0.004 0.003 0.278 注:与低难度组比较,1)P<0.05;与中难度组比较,2)P<0.05。 -
[1] KOKUDO N, TAKEMURA N, ITO K, et al. The history of liver surgery: Achievements over the past 50 years[J]. Ann Gastroenterol Surg, 2020, 4(2): 109-117. DOI: 10.1002/ags3.12322. [2] BUELL JF, CHERQUI D, GELLER DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008[J]. Ann Surg, 2009, 250(5): 825-830. DOI: 10.1097/sla.0b013e3181b3b2d8. [3] WAKABAYASHI G, CHERQUI D, GELLER DA, et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka[J]. Ann Surg, 2015, 261(4): 619-629. DOI: 10.1097/SLA.0000000000001184. [4] KOMATSU S, SCATTON O, GOUMARD C, et al. Development process and technical aspects of laparoscopic hepatectomy: Learning curve based on 15 years of experience[J]. J Am Coll Surg, 2017, 224(5): 841-850. DOI: 10.1016/j.jamcollsurg.2016.12.037. [5] BROWN KM, GELLER DA. What is the learning curve for laparoscopic major hepatectomy?[J]. J Gastrointest Surg, 2016, 20(5): 1065-1071. DOI: 10.1007/s11605-016-3100-8. [6] ABU HILAL M, ALDRIGHETTI L, DAGHER I, et al. The Southampton Consensus Guidelines for laparoscopic liver surgery: From indication to implementation[J]. Ann Surg, 2018, 268(1): 11-18. DOI: 10.1097/SLA.0000000000002524. [7] BAN D, TANABE M, ITO H, et al. A novel difficulty scoring system for laparoscopic liver resection[J]. J Hepatobiliary Pancreat Sci, 2014, 21(10): 745-753. DOI: 10.1002/jhbp.166. [8] GUO Y, LIAO R, LUO F. Establishment of surgical difficulty scoring system for laparoscopic liver resection and its application[J]. Chin J Gen Surg, 2018, 27(1): 22-28. DOI: 10.3978/j.issn.1005-6947.2018.01.004.郭杨, 廖锐, 罗放. 腹腔镜下肝切除术手术难度评分系统的建立与应用[J]. 中国普通外科杂志, 2018, 27(1): 22-28. DOI: 10.3978/j.issn.1005-6947.2018.01.004. [9] KAWAGUCHI Y, FUKS D, KOKUDO N, et al. Difficulty of laparoscopic liver resection: Proposal for a new classification[J]. Ann Surg, 2018, 267(1): 13-17. DOI: 10.1097/SLA.0000000000002176. [10] HASEGAWA Y, WAKABAYASHI G, NITTA H, et al. A novel model for prediction of pure laparoscopic liver resection surgical difficulty[J]. Surg Endosc, 2017, 31(12): 5356-5363. DOI: 10.1007/s00464-017-5616-8. [11] HALLS MC, BERARDI G, CIPRIANI F, et al. Development and validation of a difficulty score to predict intraoperative complications during laparoscopic liver resection[J]. Br J Surg, 2018, 105(9): 1182-1191. DOI: 10.1002/bjs.10821. [12] The Hepatic Surgery Group of Chinese Medical Association. Expert consensus on laparoscopic hepatectomy and guideline for operative procedure (2013 edition)[J]. Chin J Dig Surg, 2013, 12(3): 161-165. DOI: 10.3760/cma.j.issn.1673-9752.2013.03.001.中华医学会外科学分会肝脏外科学组. 腹腔镜肝切除专家共识与手术操作指南(2013版)[J]. 中华消化外科杂志, 2013, 12(3): 161-165. DOI: 10.3760/cma.j.issn.1673-9752.2013.03.001. [13] BELGHITI J, CLAVIEN P, GADZIJEV E, et al. The Brisbane 2000 terminology of liver anatomy and resections[J]. HPB, 2000, 2(3): 333-339. DOI: 10.1016/S1365-182X(17)30755-4. [14] CLAVIEN PA, BARKUN J, de OLIVEIRA ML, et al. The Clavien-Dindo classification of surgical complications: Five-year experience[J]. Ann Surg, 2009, 250(2): 187-196. DOI: 10.1097/SLA.0b013e3181b13ca2. [15] CHEN L, LI Y. The risk factors and prediction systems for posthepatectomy complications[J]. J Clin Hepatol, 2019, 35(1): 217-221. DOI: 10.3969/j.issn.1001-5256.2019.01.048.陈龙, 李钺. 肝切除术后并发症的危险因素及预测评分系统[J]. 临床肝胆病杂志, 2019, 35(1): 217-221. DOI: 10.3969/j.issn.1001-5256.2019.01.048. [16] HAN HS, SHEHTA A, AHN S, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma: Case-matched study with propensity score matching[J]. J Hepatol, 2015, 63(3): 643-650. DOI: 10.1016/j.jhep.2015.04.005. [17] CIRIA R, CHERQUI D, GELLER DA, et al. Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing[J]. Ann Surg, 2016, 263(4): 761-777. DOI: 10.1097/SLA.0000000000001413. [18] XIANG L, LI J, CHEN J, et al. Prospective cohort study of laparoscopic and open hepatectomy for hepatocellular carcinoma[J]. Br J Surg, 2016, 103(13): 1895-1901. DOI: 10.1002/bjs.10294. [19] YOON YI, KIM KH, CHO HD, et al. Long-term perioperative outcomes of pure laparoscopic liver resection versus open liver resection for hepatocellular carcinoma: A retrospective study[J]. Surg Endosc, 2020, 34(2): 796-805. DOI: 10.1007/s00464-019-06831-w. [20] ZHANG NP, ZHANG XJ. Evaluation of different hepatic blood flow blocking methods in laparoscopic hepatectomy of patients with liver cancer[J/CD]. Chin J Liver Dis (Electronic Version), 2019, 11(3): 58-63. DOI: 10.3969/j.issn.1674-7380.2019.03.011.张能平, 张雄杰. 不同肝血流阻断方式在肝癌患者腹腔镜肝切除术中的应用[J/CD]. 中国肝脏病杂志(电子版), 2019, 11(3): 58-63. DOI: 10.3969/j.issn.1674-7380.2019.03.011. [21] CIRIA R, AYLLON MD, BRICEÑO J. Difficulty scores in laparoscopic liver surgery-getting closer to a powerful and necessary tool[J]. Hepatobiliary Surg Nutr, 2019, 8(4): 428-430. DOI: 10.21037/hbsn.2019.02.11. [22] IM C, CHO JY, HAN HS, et al. Validation of difficulty scoring system for laparoscopic liver resection in patients who underwent laparoscopic left lateral sectionectomy[J]. Surg Endosc, 2017, 31(1): 430-436. DOI: 10.1007/s00464-016-4994-7. [23] LEE SY, GOH B, SEPIDEH G, et al. Laparoscopic liver resection difficulty score-a validation study[J]. J Gastrointest Surg, 2019, 23(3): 545-555. DOI: 10.1007/s11605-018-4036-y. [24] XIA AD, WANG W, BAI G, et al. Practicability of surgical difficulty scoring model for laparoscopic liver resection[J/CD]. Chin J Laparoscopic Surgery (Electronic Edition), 2020, 13(3): 166-172. DOI: 10.3877/cma.j.issn.1674-6899.2020.03.009.夏阿东, 王巍, 白光, 等. 腹腔镜肝切除评分模型可行性分析[J/CD]. 中华腔镜外科杂志(电子版), 2020, 13(3): 166-172. DOI: 10.3877/cma.j.issn.1674-6899.2020.03.009. [25] YANG J, YANG Z, JIA G, et al. Clinical practicality study of the difficulty scoring systems DSS-B and DSS-ER in laparoscopic liver resection[J]. J Laparoendosc Adv Surg Tech A, 2019, 29(1): 12-18. DOI: 10.1089/lap.2018.0150. [26] RUSSOLILLO N, MAINA C, FLERES F, et al. Comparison and validation of three difficulty scoring systems in laparoscopic liver surgery: A retrospective analysis on 300 cases[J]. Surg Endosc, 2020, 34(12): 5484-5494. DOI: 10.1007/s00464-019-07345-1. [27] RATTI F, D'ALESSANDRO V, CIPRIANI F, et al. Influence of body habitus on feasibility and outcome of laparoscopic liver resections: A prospective study[J]. J Hepatobiliary Pancreat Sci, 2016, 23(6): 373-381. DOI: 10.1002/jhbp.350. [28] TRIPKE V, HUBER T, MITTLER J, et al. Prediction of complexity and complications of laparoscopic liver surgery: The comparison of the Halls-score to the IWATE-score in 100 consecutive laparoscopic liver resections[J]. J Hepatobiliary Pancreat Sci, 2020, 27(7): 380-387. DOI: 10.1002/jhbp.731. -



 PDF下载 ( 2175 KB)
PDF下载 ( 2175 KB)

 下载:
下载:


