凝血酶原国际标准化比值/白蛋白对失代偿期肝硬化患者预后的预测价值
DOI: 10.3969/j.issn.1001-5256.2021.09.016
Value of prothrombin time-international normalized ratio to albumin ratio in predicting the prognosis of patients with decompensated cirrhosis
-
摘要:
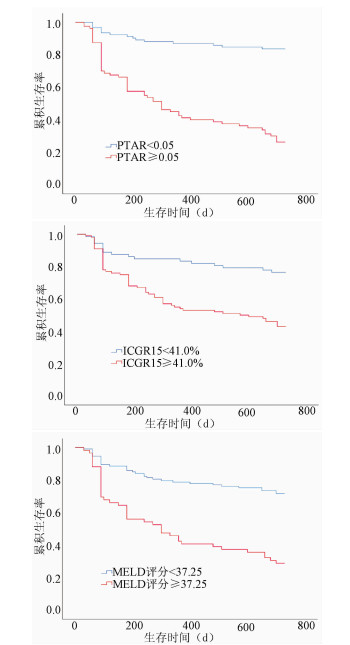
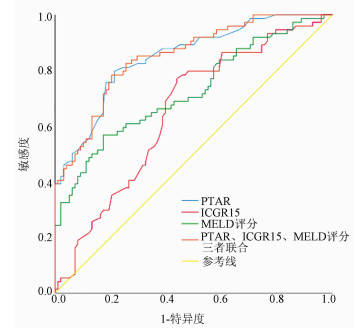
目的 探讨凝血酶原国际标准化比值/白蛋白(PTAR)评估失代偿期肝硬化患者预后的临床价值。 方法 回顾性分析2016年4月—2017年4月昆明医科大学第二附属医院收治的172例失代偿期肝硬化患者的临床资料,包括性别、年龄、病因、并发症、入院后首次的实验室指标检测等。以死亡为终点事件,根据随访2年的疾病转归情况将所纳入患者分为生存组(n=98)和死亡组(n=74)。分析影响预后的相关因素,并评估PTAR对失代偿期肝硬化患者预后的预测价值。计量资料两组间比较采用t检验或Mann-Whitney U检验。计数资料两组间比较采用χ2检验或Fisher确切概率法。对相关变量行单因素和多因素Cox回归分析。绘制受试者工作特征曲线(ROC曲线),计算曲线下面积(AUC),并根据ROC曲线的敏感度和特异度确定最佳临界值。运用Kaplan-Meier生存曲线分析不同PTAR、吲哚菁绿15 min滞留率(ICGR15)、MELD评分患者的2年生存率,并采用log-rank检验比较组间差异。 结果 死亡组患者的PTAR(Z=-7.823,P<0.001)、ICGR15(t=3.458,P=0.001)及MELD评分(t=5.921,P<0.001)均明显高于生存组。PTAR、ICGR15、MELD评分预测患者2年预后的最佳临界值分别为0.05、41.00%、37.25分,AUC分别为0.849、0.651、0.724。生存分析提示高水平PTAR组(PTAR≥0.05)患者生存率显著低于低水平PTAR组(PTAR<0.05)(χ2=60.07,P<0.001)。多因素Cox回归分析结果显示,PTAR≥0.05是患者2年死亡的独立危险因素(HR=2.564,95%CI:1.276~5.151,P=0.008)。 结论 PTAR≥0.05可作为失代偿期肝硬化患者2年死亡的独立预测因子,PTAR对失代偿肝硬化患者预后有较高的预测价值。 Abstract:Objective To investigate the value of prothrombin time-international normalized ratio to albumin ratio (PTAR) in evaluating the prognosis of patients with decompensated cirrhosis. Methods A retrospective analysis was performed for the clinical data of 172 patients with decompensated cirrhosis who were admitted to The Second Affiliated Hospital of Kunming Medical University from April 2016 to April 2017, including sex, age, etiology, complications, and first examination of laboratory markers after admission. With death as the outcome event, the patients were divided into survival group with 98 patients and death group with 74 patients according to the outcome of the disease after 2 years of follow-up. The influencing factors for prognosis were analyzed, and the value of PTAR in predicting the prognosis of patients with decompensated cirrhosis were evaluated. The t-test or the Mann-Whitney U test was used for comparison of continuous data between groups, and the chi-square test or the Fisher's exact test was used for comparison of categorical data between groups. Univariate and multivariate Cox regression analyses were performed for related variables. The receiver operating characteristic (ROC) curve was plotted and the area under the ROC curve (AUC) was calculated, and the optimal cut-off value was determined according to the sensitivity and specificity of the ROC curve. The Kaplan-Meier survival curve analysis was performed to compare 2-year survival rate between patients with different values of PTAR, indocyanine green retention rate at 15 minutes (ICGR15), and Model for End-Stage Liver Disease (MELD) score, and the log-rank test was used for comparison between groups. Results Compared with the survival group, the death group had significantly higher PTAR (Z=-7.823, P < 0.001), ICGR15 (t=3.458, P=0.001), and MELD score (t=5.921, P < 0.001). PTAR, ICGR15, and MELD score had optimal cut-off values of 0.05, 41.00%, and 37.25, respectively, in predicting 2-year prognosis, with AUCs of 0.849, 0.651, and 0.724, respectively. The survival analysis showed that the high-PTAR (PTAR≥0.05) group had a significantly lower survival rate than the low-PTAR (PTAR < 0.05) group (χ2=60.07, P < 0.001). The multivariate Cox regression analysis showed that PTAR ≥0.05 was an independent risk factor for death within 2 years (hazard ratio = 2.564, 95% confidence interval: 1.276-5.151, P=0.008). Conclusion PTAR ≥0.05 can be used as an independent predictive factor for death within 2 years in patients with decompensated cirrhosis, and PTAR has a relatively high value in predicting the prognosis of patients with decompensated cirrhosis. -
Key words:
- Liver Cirrhosis /
- Prognosis /
- Serum Albumin /
- International Normalized Ratio
-
表 1 纳入研究的两组患者临床基线特征比较
指标 死亡组(n=74) 生存组(n=98) 统计值 P值 年龄(岁) 54.81±13.64 50.51±12.44 t=2.154 0.033 男/女(例) 58/16 66/32 χ2=2.550 0.110 Alb(g/L) 26.48±4.28 33.39±5.35 t=-9.126 <0.001 ALT(U/L) 49.50(26.00~134.25) 36.00(20.50~70.00) Z=-2.787 0.005 AST(U/L) 94.00(52.75~260.75) 52.00(34.50~79.00) Z=-4.776 <0.001 GGT(U/L) 94.50(41.50~201.25) 73.00(33.50~133.00) Z=-1.761 0.078 ALP(U/L) 156.50(109.75~233.75) 121.00(92.50~157.00) Z=-3.257 0.001 TBil(μmol/L) 152.15(56.45~331.40) 74.10(35.50~160.40) Z=-3.906 <0.001 SCr(μmol/L) 74.50(61.00~90.50) 68.00(53.50~77.50) Z=-2.479 0.013 TC(mol/L) 2.33(1.76~3.60) 3.33(2.38~4.27) Z=-3.345 0.001 Na(mol/L) 137.70(133.38~139.35) 139.00(137.95~140.90) Z=-3.221 0.001 PT(s) 19.70(17.05~24.83) 15.70(13.95~17.35) Z=-6.183 <0.001 INR 1.80(1.42~2.26) 1.28(1.15~1.54) Z=-6.201 <0.001 WBC(×109/L) 6.09(3.65~7.98) 4.74(3.36~7.33) Z=-1.553 0.121 Hb(g/L) 109.36±25.87 117.66±25.26 t=-2.111 0.036 中性粒细胞计数(×109/L) 4.43(2.10~5.64) 2.79(2.05~4.97) Z=-1.803 0.071 PTAR 0.06(0.05~0.09) 0.04(0.03~0.05) Z=-7.823 <0.001 ICGR15(%) 47.58±16.86 37.96±18.91 t=3.458 0.001 MELD评分(分) 38.15±9.04 31.11±6.56 t=5.921 <0.001 静脉曲张[例(%)] 47(63.5) 67(68.4) χ2=0.444 0.505 静脉曲张破裂出血[例(%)] 28(37.8) 19(19.4) χ2=7.227 0.007 肝性脑病[例(%)] 14(18.9) 9(9.2) χ2=3.450 0.063 腹腔积液[例(%)] 62(83.8) 49(50.0) χ2=21.026 <0.001 细菌感染[例(%)] 34(45.9) 41(41.8) χ2=0.290 0.591 肝肾综合征[例(%)] 3(4.1) 2(2.0) 0.653 表 2 3种指标预测失代偿期肝硬化患者2年内死亡的诊断效能比较
指标 AUC 95% CI 敏感度 特异度 最佳临界值 约登指数 PTAR 0.849 0.791~0.906 0.797 0.786 0.05 0.583 ICGR15 0.651 0.569~0.733 0.770 0.561 41.00% 0.331 MELD评分 0.724 0.646~0.802 0.568 0.827 37.25分 0.395 三者联合 0.851 0.795~0.907 0.784 0.796 0.41 0.580 表 3 单因素及多因素Cox回归分析
指标 单因素分析 多因素分析 B值 P值 HR 95%CI B值 P值 HR 95%CI 年龄 0.019 0.051 1.019 1.000~1.039 ICGR15 0.021 0.001 1.021 1.009~1.034 MELD评分 0.070 <0.001 1.073 1.047~1.099 Hb -0.010 0.044 0.990 0.981~1.000 Alb -0.177 <0.001 0.838 0.799~0.878 -0.121 <0.001 0.886 0.835~0.939 PTAR≥0.05 1.923 <0.001 6.839 3.861~12.114 0.941 0.008 2.564 1.276~5.151 ALT 0.002 <0.001 1.002 1.001~1.002 AST 0.002 <0.001 1.002 1.001~1.003 0.001 <0.001 1.001 1.001~1.002 ALP 0.001 0.020 1.001 1.000~1.001 TBil 0.004 <0.001 1.004 1.002~1.005 SCr 0.002 0.082 1.002 1.000~1.004 TC -0.210 0.025 0.811 0.675~0.974 PT 0.096 <0.001 1.101 1.070~1.132 INR 0.813 <0.001 2.255 1.768~2.876 静脉曲张破裂出血 0.623 0.009 1.865 1.165~2.984 腹腔积液 1.301 <0.001 3.647 1.977~6.829 -
[1] European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis[J]. J Hepatol, 2018, 69(2): 406-460. DOI: 10.1016/j.jhep.2018.03.024. [2] BERNARDI M, CARACENI P. Novel perspectives in the management of decompensated cirrhosis[J]. Nat Rev Gastroenterol Hepatol, 2018, 15(12): 753-764. DOI: 10.1038/s41575-018-0045-2. [3] KOKUDO T, HASEGAWA K, AMIKURA K, et al. Assessment of preoperative liver function in patients with hepatocellular carcinoma-the Albumin-Indocyanine Green Evaluation (ALICE) grade[J]. PLoS One, 2016, 11(7): e0159530. DOI: 10.1371/journal.pone.0159530. [4] WANG YY, ZHAO XH, MA L, et al. Comparison of the ability of Child-Pugh score, MELD score, and ICG-R15 to assess preoperative hepatic functional reserve in patients with hepatocellular carcinoma[J]. J Surg Oncol, 2018, 118(3): 440-445. DOI: 10.1002/jso.25184. [5] CHENG XP, ZHAO J, CHEN Y, et al. Comparison of the ability of the PDD-ICG clearance test, CTP, MELD, and MELD-Na to predict short-term and medium-term mortality in patients with decompensated hepatitis B cirrhosis[J]. Eur J Gastroenterol Hepatol, 2016, 28(4): 444-448. DOI: 10.1097/MEG.0000000000000538. [6] HARUKI K, SHIBA H, SAITO N, et al. Risk stratification using a novel liver functional reserve score of combination prothrombin time-international normalized ratio to albumin ratio and albumin in patients with hepatocellular carcinoma[J]. Surgery, 2018, 164(3): 404-410. DOI: 10.1016/j.surg.2018.02.022. [7] GAO F, CAI MX, LIN MT, et al. Prognostic value of international normalized ratio to albumin ratio among critically ill patients with cirrhosis[J]. Eur J Gastroenterol Hepatol, 2019, 31(7): 824-831. DOI: 10.1097/MEG.0000000000001339. [8] Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35(11): 2408-2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006.中华医学会肝病学分会. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35(11): 2408-2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. [9] SHAH AS, AMARAPURKAR DN. Natural history of cirrhosis of liver after first decompensation: A prospective study in India[J]. J Clin Exp Hepatol, 2018, 8(1): 50-57. DOI: 10.1016/j.jceh.2017.06.001. [10] DURAND F, VALLA D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD[J]. J Hepatol, 2005, 42(Suppl 1): s100-s107. DOI: 10.1016/j.jhep.2004.11.015. [11] KAMATH PS, WIESNER RH, MALINCHOC M, et al. A model to predict survival in patients with end-stage liver disease[J]. Hepatology, 2001, 33(2): 464-470. DOI: 10.1053/jhep.2001.22172. [12] WIESNER R, EDWARDS E, FREEMAN R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers[J]. Gastroenterology, 2003, 124(1): 91-96. DOI: 10.1053/gast.2003.50016. [13] ZHAI YZ, YUE YY, DING DP, et al. The value of serum prealbumin combined with MELD score in predicting the prognosis of patients with decompensated cirrhosis[J]. Chin J Hepatol, 2017, 25(7): 533-535. DOI: 10.3760/cma.j.issn.1007-3418.2017.07.012.翟永贞, 岳阳阳, 丁德平, 等. 血清前白蛋白联合终末期肝病模型评分评估失代偿期肝硬化患者预后的临床价值[J]. 中华肝脏病杂志, 2017, 25(7): 533-535. DOI: 10.3760/cma.j.issn.1007-3418.2017.07.012. [14] JIANG M, LIU F, XIONG WJ, et al. Combined MELD and blood lipid level in evaluating the prognosis of decompensated cirrhosis[J]. World J Gastroenterol, 2010, 16(11): 1397-1401. DOI: 10.3748/wjg.v16.i11.1397. [15] DU HL, HE HY, XIAO CJ, et al. Value of indocyanine green clearance test in predicting the short-term prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure[J]. J Clin Hepatol, 2019, 35(12): 2759-2764. DOI: 10.3969/j.issn.1001-5256.2019.12.024.都泓莲, 何鸿雁, 肖慈君, 等. 吲哚菁绿清除试验对HBV相关慢加急性肝衰竭患者短期预后的评估[J]. 临床肝胆病杂志, 2019, 35(12): 2759-2764. DOI: 10.3969/j.issn.1001-5256.2019.12.024. [16] QIN H, WAN H, WU XQ, et al. Indocyanine green clearance test assessment of liver reserve function and estimation of prognosis in patients with cirrhosis and liver failure[J]. Chin J Hepatol, 2015, 23(7): 540-542. DOI: 10.3760/cma.j.issn.1007-3418.2015.07.015.秦华, 万红, 吴晓庆, 等. 吲哚菁绿清除试验对肝硬化及肝衰竭患者肝脏储备功能的评估及预后的判断[J]. 中华肝脏病杂志, 2015, 23(7): 540-542. DOI: 10.3760/cma.j.issn.1007-3418.2015.07.015. [17] STAUBER RE, WAGNER D, STADLBAUER V, et al. Evaluation of indocyanine green clearance and model for end-stage liver disease for estimation of short-term prognosis in decompensated cirrhosis[J]. Liver Int, 2009, 29(10): 1516-1520. DOI: 10.1111/j.1478-3231.2009.02104.x. [18] STOCKMANN M, MALINOWSKI M, LOCK JF, et al. Factors influencing the indocyanine green (ICG) test: Additional impact of acute cholestasis[J]. Hepatogastroenterology, 2009, 56(91-92): 734-738. [19] IMAMURA H, SANO K, SUGAWARA Y, et al. Assessment of hepatic reserve for indication of hepatic resection: Decision tree incorporating indocyanine green test[J]. J Hepatobiliary Pancreat Surg, 2005, 12(1): 16-22. DOI: 10.1007/s00534-004-0965-9. [20] LONGHEVAL G, VEREERSTRAETEN P, THIRY P, et al. Predictive models of short- and long-term survival in patients with nonbiliary cirrhosis[J]. Liver Transpl, 2003, 9(3): 260-267. DOI: 10.1053/jlts.2003.50049. [21] KANG N, QI LC, YUAN Y, et al. Clinical significance of PTAR, Child-Pugh and MELD score in predicting the occurence of acute-on-chronic liver failure in patients with cirrhosis[J]. Chin J Gastroenterol Hepatol, 2020, 29(10): 1171-1178. DOI: 10.3969/j.issn.1006-5709.2020.10.019.康宁, 齐丽翠, 袁岳, 等. PTAR联合Child-Pugh及MELD评分对肝硬化患者发生慢加急性肝衰竭预测价值研究[J]. 胃肠病学和肝病学杂志, 2020, 29(10): 1171-1178. DOI: 10.3969/j.issn.1006-5709.2020.10.019. [22] KANG N, WANG CK, QI LC, et al. Clinical significance of PTAR and ICGR15 joint score in predicting the occurrence of acute-on-chronic liver failure in patients with hepatitis B cirrhosis[J]. Chin J Pract Intern Med, 2020, 40(7): 558-562. DOI: 10.19538/j.nk2020070108.康宁, 王存凯, 齐丽翠, 等. PTAR联合ICGR15对乙肝肝硬化患者发生慢加急性肝衰竭的预测价值研究[J]. 中国实用内科杂志, 2020, 40(7): 558-562. DOI: 10.19538/j.nk2020070108. -



 PDF下载 ( 2730 KB)
PDF下载 ( 2730 KB)

 下载:
下载: