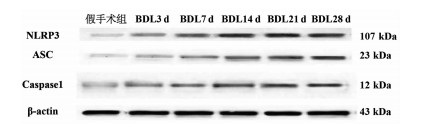
NLRP3炎性体在胆总管结扎诱导的肝纤维化大鼠模型中的表达特点
DOI: 10.3969/j.issn.1001-5256.2021.09.020
Expression of NLRP3 inflammatory body in a rat model of liver fibrosis induced by common bile duct ligation
-
摘要:
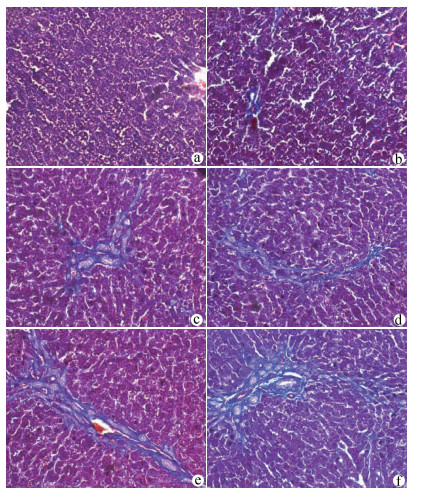
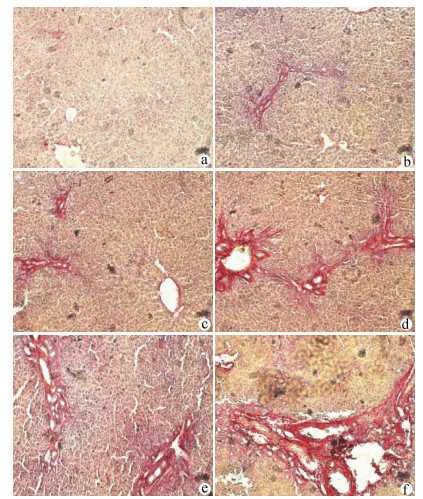
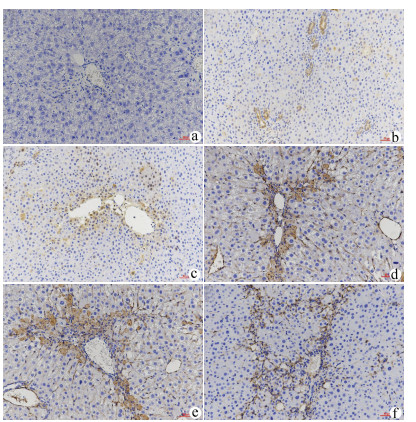
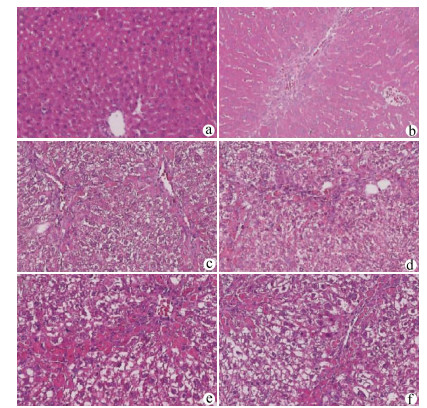
目的 探讨NLRP3炎性体在胆总管结扎(BDL)大鼠肝纤维化过程中的表达特点,探索NLRP3炎性体与肝纤维化的关系。 方法 将65只SD大鼠随机分为假手术组(n=15)、BDL模型组(n=50)。分别于第3、7、14、21、28天,处死10只模型组大鼠,同时处死3只假手术组大鼠。检测血清ALT、AST、DBil、TBil、TBA、ALP水平,对肝组织行HE、Masson、天狼星红-苦味酸染色,评估各组肝纤维化程度,免疫组化检测肝组织α平滑肌肌动蛋白(αSMA)、TGFβ1的表达水平,Western Blot、qRT-PCR检测肝组织NLRP3炎性体的表达水平,ELISA法检测肝组织炎症IL-1β水平。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 与假手术组比较,各BDL模型组大鼠血清ALT、AST、DBil、TBil、TBA、ALP水平均升高(P值均<0.05),肝组织IL-1β水平均升高(P值均<0.05),并于第3天达到最高,其后有所降低。与假手术组比较,随时间延长,BDL大鼠模型肝纤维化评分明显增加(P值均<0.05),免疫组化显示αSMA、TGFβ1表达逐渐增加(P值均<0.05),Western Blot、qRT-PCR显示肝组织NLRP3炎性体蛋白表达逐渐增加(P值均<0.05),并于第14天后保持稳定。 结论 BDL大鼠模型肝功能损伤持续存在,肝组织病理学存在肝炎-肝纤维化-肝硬化的动态演变,NLRP3炎性体处于持续活化状态,可能在肝纤维化过程中发挥重要作用。 -
关键词:
- 肝硬化 /
- 胆汁淤积 /
- NLR家族, 热蛋白结构域包含蛋白3 /
- 大鼠, Sprague-Dawley
Abstract:Objective To investigate the expression of NLRP3 inflammatory body in the process of liver fibrosis in a rat model of common bile duct ligation (BDL) and the association of NLRP3 inflammatory body with liver fibrosis. Methods A total of 65 Sprague-Dawley rats were randomly divided into sham-operation group with 15 rats and BDL model group with 50 rats. On days 3, 7, 14, 21, and 28, 10 rats in the model group and 3 rats in the sham-operation group were sacrificed. The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), direct bilirubin (DBil), total bilirubin (TBil), total bile acid (TBA), and alkaline phosphatase (ALP) were measured, and HE staining, Masson staining, and sirius red-picric acid staining were performed for liver tissue to evaluate liver fibrosis degree. Immunohistochemistry was used to measure the expression levels of alpha-smooth muscle actin (α-SMA) and transforming growth factor-β1 (TGF-β1) in liver tissue, Western blot and qRT-PCR were used to measure the expression level of NLRP3 inflammatory body, and ELISA was used to measure the level of the inflammatory factor interleukin-1β (IL-1β) in liver tissue. An analysis of variance was used for comparison of continuous data between groups, and the least significant difference t-test was used for further comparison between two groups. Results Compared with the sham-operation group, the BDL model group had significant increases in the serum levels of ALT, AST, DBil, TBil, TBA, and ALP (all P < 0.05) and the level of IL-1β in liver tissue (P < 0.05), which reached the highest level on day 3 and then decreased. Compared with the sham-operation group over time, the BDL group had a significant increase in liver fibrosis score (P < 0.05); immunohistochemistry showed gradual increases in the expression of SMA-α and TGF-β1 (P < 0.05), and Western blot and qRT-PCR showed a gradual increase in the protein expression of NLRP3 inflammatory body in liver tissue (P < 0.05), which remained stable after day 14. Conclusion Liver injury exists persistently in a rat model of BDL, and liver histopathology shows the dynamic evolution of hepatitis, liver fibrosis, and liver cirrhosis. NLRP3 inflammatory body is in a state of continuous activation and may play an important role in the process of liver fibrosis. -
表 1 各组大鼠血清AST、ALT、ALP、DBil、TBA、TBil指标比较
组别 动物数(只) AST(U/L) ALT(U/L) ALP(U/L) DBil(μmol/L) TBA(μmol/L) TBil(μmol/L) 假手术组 15 91.05±6.68 30.60±13.03 169.70±37.65 0.07±0.08 16.68±5.25 0.42±0.13 BDL3 d 10 808.80±159.91) 154.00±63.771) 666.40±139.701) 141.60±24.221) 383.60±185.101) 151.60±32.611) BDL7 d 10 233.10±11.861)2) 31.40±5.681)2) 388.20±74.171)2) 61.66±15.401)2) 122.30±19.511)2) 71.51±16.261)2) BDL14 d 10 234.20±12.081)2) 25.10±9.191)2) 347.70±63.381)2) 70.42±25.001)2) 136.20±26.021)2) 72.78±24.281)2) BDL21 d 10 237.90±9.621)2) 41.10±12.111)2) 408.40±61.851)2) 74.14±11.051)2) 140.00±21.651)2) 77.21±22.151)2) BDL28 d 10 237.30±9.711)2) 63.10±23.871)2)4) 466.80±109.001)2)4) 84.19±13.661)2) 141.50±73.971)2) 88.96±9.931)2)3) F值 170.9 31.49 43.92 94.50 26.54 71.10 P值 <0.01 <0.01 <0.01 <0.01 <0.01 <0.01 注:与假手术组相比,1)P<0.05;与BDL3 d相比,2)P<0.05;与BDL7 d相比,3)P<0.05; 与BDL14 d相比,4)P<0.05。 表 2 免疫组化半定量及肝纤维化评分
组别 动物数(只) αSMA表达强度(IOD/area)×100 TGFβ1表达强度(IOD/area)×100 肝纤维化评分 假手术组 15 1.16±0.42 1.05±0.38 0.00±0.00 BDL3 d 10 1.75±0.73 1.59±0.57 1.30±0.671) BDL7 d 10 4.04±0.771)2) 4.35±1.071)2) 2.30±0.951)2) BDL14 d 10 5.99±0.781)2)3) 6.70±0.991)2)3) 3.60±1.171)2)3) BDL21 d 10 7.12±0.611)2)3)4) 7.92±1.381)2)3)4) 4.80±1.401)2)3)4) BDL28 d 10 8.31±1.761)2)3)4)5) 9.11±1.551)2)3)4)5) 5.10±1.201)2)3)4) F值 114.60 118.90 51.41 P值 <0.01 <0.01 <0.01 注:与假手术组相比,1)P<0.05;与BDL3 d相比,2)P<0.05;与BDL7 d相比,3)P<0.05; 与BDL14 d相比,4)P<0.05;与BDL21 d相比,5)P<0.05。 表 3 NLRP3炎性体蛋白表达
组别 动物数(只) NLRP3(NLRP3/β-actin) ASC(ASC/β-actin) caspase1(caspase1/β-actin) 假手术组 15 1 1 1 BDL3 d 10 1.18±0.07 1.78±0.121) 1.74±0.141) BDL7 d 10 1.60±0.171)2) 2.18±0.151)2) 2.35±0.131)2) BDL14 d 10 2.07±0.141)2)3) 2.71±0.171)2)3) 2.80±0.161)2)3) BDL21 d 10 2.21±0.161)2)3) 2.76±0.181)2)3) 2.82±0.161)2)3) BDL28 d 10 2.24±0.131)2)3) 2.78±0.161)2)3) 2.78±0.171)2)3) F值 55.90 73.89 89.55 P值 <0.01 <0.01 <0.01 注:与假手术组相比,1)P<0.05;与BDL3 d相比,2)P<0.05;与BDL7 d相比,3)P<0.05。 表 4 NLRP3炎性体mRNA表达
组别 动物数(只) NLRP3(NLRP3/β-actin) ASC(ASC/β-actin) caspase1(caspase1/β-actin) 假手术组 15 1 1 1 BDL3 d 10 1.73±0.181) 1.59±0.221) 1.52±0.191) BDL7 d 10 2.16±0.321)2) 2.12±0.291)2) 1.80±0.241)2) BDL14 d 10 3.03±0.321)2)3) 2.74±0.291)2)3) 2.61±0.241)2)3) BDL21 d 10 2.97±0.231)2)3) 2.78±0.231)2)3) 2.71±0.241)2)3) BDL28 d 10 3.03±0.181)2)3) 2.79±0.201)2)3) 2.77±0.191)2)3) F值 177.50 141.20 173.00 P值 <0.01 <0.01 <0.01 注:与假手术组相比,1)P<0.05;与BDL3 d相比,2)P<0.05;与BDL7 d相比,3)P<0.05。 表 5 各组肝组织IL-1β水平
组别 动物数(只) IL-1β(pg/mg) 假手术组 15 45.81±4.77 BDL3 d 10 139.90±18.381) BDL7 d 10 55.83±10.251)2) BDL14 d 10 79.76±12.921)2)3) BDL21 d 10 70.52±8.621)2)3) BDL28 d 10 100.60±8.691)2)3)4)5) F值 106.10 P值 <0.01 注:与假手术组相比,1)P<0.05;与BDL3 d相比,2)P<0.05;与BDL7 d相比,3)P<0.05;与BDL14 d相比,4)P<0.05;与BDL21 d相比,5)P<0.05。 -
[1] DU QH, ZHANG CJ, LI WH, et al. Gan Shen Fu Fang ameliorates liver fibrosis in vitro and in vivo by inhibiting the inflammatory response and extracellular signal-regulated kinase phosphorylation[J]. World J Gastroenterol, 2020, 26(21): 2810-2820. DOI: 10.3748/wjg.v26.i21.2810. [2] TRAUTWEIN C, FRIEDMAN SL, SCHUPPAN D, et al. Hepatic fibrosis: Concept to treatment[J]. J Hepatol, 2015, 62(1 Suppl): s15-s24. DOI: 10.1016/j.jhep.2015.02.039. [3] WU X, DONG L, LIN X, et al. Relevance of the NLRP3 inflammasome in the pathogenesis of chronic liver disease[J]. Front Immunol, 2017, 8: 1728. DOI: 10.3389/fimmu.2017.01728. [4] HE K, ZHU X, LIU Y, et al. Inhibition of NLRP3 inflammasome by thioredoxin-interacting protein in mouse Kupffer cells as a regulatory mechanism for non-alcoholic fatty liver disease development[J]. Oncotarget, 2017, 8(23): 37657-37672. DOI: 10.18632/oncotarget.17489. [5] CUI K, YAN G, XU C, et al. Invariant NKT cells promote alcohol-induced steatohepatitis through interleukin-1β in mice[J]. J Hepatol, 2015, 62(6): 1311-1318. DOI: 10.1016/j.jhep.2014.12.027. [6] WU X, ZHANG F, XIONG X, et al. Tetramethylpyrazine reduces inflammation in liver fibrosis and inhibits inflammatory cytokine expression in hepatic stellate cells by modulating NLRP3 inflammasome pathway[J]. IUBMB Life, 2015, 67(4): 312-321. DOI: 10.1002/iub.1348. [7] LENTSCH AB, KATO A, YOSHIDOME H, et al. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury[J]. Hepatology, 2000, 32(2): 169-173. DOI: 10.1053/jhep.2000.9323. [8] SHARAWY MH, ABDEL-RAHMAN N, MEGAHED N, et al. Paclitaxel alleviates liver fibrosis induced by bile duct ligation in rats: Role of TGFβ1, IL-10 and c-Myc[J]. Life Sci, 2018, 211: 245-251. DOI: 10.1016/j.lfs.2018.09.037. [9] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Consensus on the diagnosis and therapy of hepatic fibrosis(2019)[J]. J Clin Hepatol, 2019, 35(10): 2163-2172. DOI: 10.3969/j.issn.1001-5256.2019.10.007.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会感染病学分会. 肝纤维化诊断及治疗共识(2019年)[J]. 临床肝胆病杂志, 2019, 35(10): 2163-2172. DOI: 10.3969/j.issn.1001-5256.2019.10.007. [10] TAG CG, SAUER-LEHNEN S, WEISKIRCHEN S, et al. Bile duct ligation in mice: Induction of inflammatory liver injury and fibrosis by obstructive cholestasis[J]. J Vis Exp, 2015, 96: 52438. DOI: 10.3791/52438. [11] ELPEK GÖ. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update[J]. World J Gastroenterol, 2014, 20(23): 7260-7276. DOI: 10.3748/wjg.v20.i23.7260. [12] ZHOU WC, ZHANG QB, QIAO L. Pathogenesis of liver cirrhosis[J]. World J Gastroenterol, 2014, 20(23): 7312-7324. DOI: 10.3748/wjg.v20.i23.7312. [13] CHANG J, LAN T, LI C, et al. Activation of Slit2-Robo1 signaling promotes liver fibrosis[J]. J Hepatol, 2015, 63(6): 1413-1420. DOI: 10.1016/j.jhep.2015.07.033. [14] YANG F, LI LH. Effect of vitamin D receptor activation on hepatic fibrosis induced by bile duct ligation in mice and its mechanism[J]. J Jilin Univ(Med Edit), 2020, 46(4): 722-727. DOI: 10.13481/j.1671-587x.20200409.杨帆, 李丽华. 维生素D受体激活对小鼠胆管结扎所致肝纤维化的影响及其机制[J]. 吉林大学学报(医学版), 2020, 46(4): 722-727. DOI: 10.13481/j.1671-587x.20200409. [15] WU N, MENG F, ZHOU T, et al. The secretin/secretin receptor axis modulates ductular reaction and liver fibrosis through changes in transforming growth factor-β1-mediated biliary senescence[J]. Am J Pathol, 2018, 188(10): 2264-2280. DOI: 10.1016/j.ajpath.2018.06.015. [16] GRESSNER AM, WEISKIRCHEN R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets[J]. J Cell Mol Med, 2006, 10(1): 76-99. DOI: 10.1111/j.1582-4934.2006.tb00292.x. [17] CAO Z, WANG Y, LONG Z, et al. Interaction between autophagy and the NLRP3 inflammasome[J]. Acta Biochim Biophys Sin (Shanghai), 2019, 51(11): 1087-1095. DOI: 10.1093/abbs/gmz098. [18] GROSLAMBERT M, PY BF. Spotlight on the NLRP3 inflammasome pathway[J]. J Inflamm Res, 2018, 11: 359-374. DOI: 10.2147/JIR.S141220. [19] LAMKANFI M, DIXIT VM. Mechanisms and functions of inflammasomes[J]. Cell, 2014, 157(5): 1013-1022. DOI: 10.1016/j.cell.2014.04.007. [20] STROWIG T, HENAO-MEJIA J, ELINAV E, et al. Inflammasomes in health and disease[J]. Nature, 2012, 481(7381): 278-286. DOI: 10.1038/nature10759. [21] INZAUGARAT ME, JOHNSON CD, HOLTMANN TM, et al. NLR family pyrin domain-containing 3 inflammasome activation in hepatic stellate cells induces liver fibrosis in mice[J]. Hepatology, 2019, 69(2): 845-859. DOI: 10.1002/hep.30252. [22] WANG H, LV C, WANG S, et al. NLRP3 Inflammasome involves in the acute exacerbation of patients with chronic obstructive pulmonary disease[J]. Inflammation, 2018, 41(4): 1321-1333. DOI: 10.1007/s10753-018-0780-0. [23] WADA J, MAKINO H. Innate immunity in diabetes and diabetic nephropathy[J]. Nat Rev Nephrol, 2016, 12(1): 13-26. DOI: 10.1038/nrneph.2015.175. [24] LIU D, ZENG X, LI X, et al. Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases[J]. Basic Res Cardiol, 2018, 113(1): 5. DOI: 10.1007/s00395-017-0663-9. [25] ZHEN Y, ZHANG H. NLRP3 Inflammasome and inflammatory bowel disease[J]. Front Immunol, 2019, 10: 276. DOI: 10.3389/fimmu.2019.00276. [26] ZHANG N, YAN N, ZHOU ME, et al. Development and treatment of NLRP3 inflammatory corpuscule in acute and chronic liver diseases[J]. Liaoning J Trad Chin Med, 2020, 47(5): 217-220. DOI: 10.13192/j.issn.1000-1719.2020.05.065.张娜, 晏旎, 周蒙恩, 等. NLRP3炎症小体在急慢性肝脏疾病发生和治疗中的研究进展[J]. 辽宁中医杂志, 2020, 47(5): 217-220. DOI: 10.13192/j.issn.1000-1719.2020.05.065. [27] MRIDHA AR, WREE A, ROBERTSON A, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice[J]. J Hepatol, 2017, 66(5): 1037-1046. DOI: 10.1016/j.jhep.2017.01.022. [28] GONG Z, ZHOU J, ZHAO S, et al. Chenodeoxycholic acid activates NLRP3 inflammasome and contributes to cholestatic liver fibrosis[J]. Oncotarget, 2016, 7(51): 83951-83963. DOI: 10.18632/oncotarget.13796. -



 PDF下载 ( 7845 KB)
PDF下载 ( 7845 KB)

 下载:
下载: