BCLC 0/A期肝细胞癌根治性切除术后复发评分预警系统的建立及其预测价值分析
DOI: 10.3969/j.issn.1001-5256.2021.09.022
Establishment and predictive value of an early warning system for recurrence after radical resection of BCLC stage 0/A hepatocellular carcinoma
-
摘要:
目的 建立BCLC 0/A期肝细胞癌(HCC)根治性切除术后复发评分预警系统(PLC-EWSPRS)并对其预测价值进行分析。 方法 回顾性分析川北医学院附属医院2009年1月—2015年1月行根治性切除的232例BCLC 0/A期HCC患者的临床资料。根据电话或门诊资料随访术后5年内是否复发分为复发组(103例)和非复发组(129例)。符合正态分布的计量资料两组间比较采用t检验; 非正态分布的计量资料两组间比较采用Mann-Whitney U检验。计数资料两组间采用χ2检验或Fisher精确法检验。将差异具有统计学意义的指标纳入二元logistic回归分析,探讨BCLC 0/A期HCC术后复发的危险因素。将独立危险因素赋值为2分,危险因素赋值为1分,建立PLC-EWSPRS系统。采用受试者工作特征(ROC)曲线下面积(AUC)评估其诊断效能。 结果 两组患者术前AST(Z=3.864, P<0.001)、ALT(Z=4.587, P<0.001)复发组明显高于非复发组,术前Alb(t=-5.628, P<0.001)复发组明显低于非复发组; 复发组HBsAg阳性、包膜侵犯、微血管侵犯(MVI)、肿瘤直径≥5 cm、肝硬化(中重度)、非R0切除、5年死亡数所占比例均显著高于非复发组(χ2值分别为35.539、22.325、13.398、7.130、4.312、4.034、18.527,P值均<0.05)。回归分析显示:术前Alb<40 g/L(OR=5.796,P<0.001)、术前ALT≥40 U/L(OR=3.029,P=0.002)、MVI(OR=3.981,P=0.003)、HBsAg阳性(OR=7.829,P<0.001)、包膜侵犯(OR=5.357,P<0.001)、非R0切除(OR=3.048,P=0.018)均为根治性切除的BCLC 0/A期HCC术后5年复发的独立危险因素。根据PLC-EWSPRS系统拟定的赋分标准,复发组最低分2分,最高分14分; 非复发组最低分0分,最高分11分,复发组分值明显高于非复发组,两组患者分值比较差异具有统计学意义(P<0.05)。ROC曲线分析结果显示,预测根治性切除的BCLC 0/A期HCC者术后5年内复发的AUC(95%CI)为0.918(0.883~0.953),P<0.001;亚组预测低、中、高分数段术后5年内复发的AUC(95%CI)分别为:0.796(0.695~0.896),P=0.002;0.859(0.791~0.927),P<0.001;0.944(0.839~1.000),P=0.044。 结论 PLC-EWSPRS对BCLC 0/A期HCC患者术后5年复发具有良好的预测价值,对根治性切除的BCLC 0/A期HCC患者术后复查及治疗策略的制订具有重要的指导意义。 Abstract:Objective To establish an Early Warning System for Recurrence Scoring after Radical Resection of BCLC stage 0/A Primary Liver Cancer (PLC-EWSPRS), and to investigate its predictive value. Methods A retrospective analysis was performed for the clinical data of 232 patients with BCLC stage 0/A liver cancer who underwent radical resection in Affiliated Hospital of Chuanbei Medical College from January 2009 to January 2015, and according to the presence or absence of recurrence within 5 years after surgery based on telephone or outpatient follow-up data, the patients were divided into recurrence group with 103 patients and non-recurrence group with 129 patients. The t-test was used for comparison of normally distributed continuous data between groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between groups; the chi-square test or Fisher's exact test was used for comparison of categorical data between groups. The indices with statistical significance were included in the binary logistic regression analysis to investigate the risk factors for recurrence of BCLC stage 0/A liver cancer after surgery. Two points were assigned for independent risk factors and one point was assigned for risk factors to establish the PLC-EWSPRS system. The receiver operating characteristic (ROC) curve and the area under the ROC curve (AUC) were used to evaluate the diagnostic efficiency of this system. Results Compared with the non-recurrence group, the recurrence group had significantly higher levels of aspartate aminotransferase and alanine aminotransferase (ALT) and a significantly lower level of albumin (Alb) before surgery (Z=3.864 and 4.587, t=-5.628, all P < 0.001), as well as a significantly higher proportion of patients with positive HBsAg, capsular invasion, microvascular invasion (MVI), tumor diameter ≥5 cm, liver cirrhosis (moderate-to-severe), non-R0 resection, or death within 5 years (χ2=35.539, 22.325, 13.398, 7.130, 4.312, 4.034, and 18.527, all P < 0.05). The regression analysis showed that preoperative Alb < 40 g/L (odds ratio [OR]=5.796, P < 0.001), preoperative ALT ≥40 U/L (OR=3.029, P=0.002), MVI (OR=3.981, P=0.003), positive HBsAg (OR=7.829, P< 0.001), capsular invasion (OR=5.357, P< 0.001), and non-R0 resection (OR=3.048, P=0.018) were independent risk factors for recurrence of BCLC stage 0/A liver cancer within 5 years after surgery. According to the assignment criteria of the PLC-EWSPRS system, the recurrence group had the lowest score of 2 points and the highest score of 14 points, while the non-recurrence had the lowest score of 0 point and the highest score of 11 points, and the recurrence group had a significantly higher score than the non-recurrence group (P < 0.05). The ROC curve analysis showed that the PLC-EWSPRS system had an AUC of 0.918 (95% confidence interval [CI]: 0.883-0.953, P < 0.001) in predicting recurrence within 5 years after surgery in patients with BCLC stage 0/A liver cancer undergoing radical resection, and subgroup analysis showed that the system had an AUC of 0.796 (95% CI: 0.695-0.896, P=0.002), 0.859 (95% CI: 0.791-0.927, P < 0.001), and 0.944 (95% CI: 0.839-1.000, P=0.044), respectively, in predicting recurrence within 5 years after surgery in patients with a low score of 0-5 points, a moderate score of 6-10 points, and a high score of 11-14 points. Conclusion The PLC-EWSPRS system has a good value in predicting the recurrence of BCLC stage 0/A liver cancer within 5 years after surgery and thus has important guiding significance for postoperative reexamination and treatment strategy for patients with BCLC stage 0/A liver cancer undergoing radical resection. -
Key words:
- Carcinoma, Hepatocellular /
- Hepatectomy /
- Recurrence /
- Risk Factors
-
原发性肝癌(primary liver cancer,PLC)是癌症相关死亡的最主要因素之一,2018最新的GLOBOCAN统计显示其占所有癌症死亡8.2%[1]。其中,肝细胞癌(HCC)占90%以上,因起病隐匿,发现较晚,预后欠佳[2]。根据BCLC分期诊疗指南,0/A期肝癌优先考虑手术切除治疗,而稍晚期的则推荐其考虑经肝动脉化疗栓塞(TACE)治疗,但疗效差异较大[3]。因此,早期发现并行根治性切除是HCC患者获得较长总体生存最重要的治疗方法。然而,术后复发转移却极大影响疗效,研究[4]表明术后5年复发率高达70%,而且目前尚无针对复发后治疗优良方案,导致长期存活率低。因此,如何预测术后患者复发风险,建立相关的预警系统来指导患者术后复查及治疗显得尤其重要。根据预测系统为患者拟定个体化复诊方案,以达到早期治疗、分类干预、降低复发率从而提高生存率的目的。以期为根治性切除BCLC 0/A期肝癌患者获得更好的术后生存提供参考。
1. 资料与方法
1.1 研究对象
回顾性分析2009年1月—2015年1月在川北医学院附属医院接受根治性外科切除术治疗的BCLC 0/A期HCC患者相关资料。纳入标准:(1)根据美国肝病学会(AASLD)和欧洲肝病学会(EASL)肝癌治疗指南诊断为BCLC 0/A期的HCC患者,并在第一次诊断后即接受手术切除; (2) Child-Pugh A~B级、ECOG-PS 0分; (3)影像学检查明确未见远处转移。排除标准:(1)BCLC分期更晚者; (2)既往有微波及射频消融、放化疗、靶向药物等相关治疗史者; (3) 伴有严重的心、肺、肾功能不全或其他系统恶性肿瘤者; (4)临床资料不全及失访者。
1.2 治疗方式
开腹手术与腹腔镜下手术的选择,在以能更好地达到根治性切除的前提下,根据临床主刀医师的经验及习惯进行选择。所纳入病例的手术均由经验丰富的教授主刀完成(主刀台次>100例同类手术)。本研究中根治性外科切除术定义:完全切除肿瘤结节,主要采取解剖性切除,不能行解剖性切除的要求至少达到无肉眼可见肿瘤切缘。根据Couinaud的分类,解剖性切除是指对肝功能储备满意的患者进行至少一个节段(即节段切除、扇形切除或半肝切除)的完全切除。非R0切除患者术后予以口服中成药(半年至一年):十味蒂达胶囊0.9 g(2粒)每天3次、复方斑蝥胶囊750 mg(3粒)每天2次; 术前HBsAg阳性患者且病毒滴度高的患者,术前行抗病毒治疗待病毒滴度下降后行手术治疗; 所有术前HBsAg阳性患者术后长期予以抗病毒治疗(恩替卡韦0.5 mg每天1次)。复发前,余未行其他方式治疗,门诊定期复查随访。
1.3 数据收集
收集入组患者的一般人口学资料(年龄、性别、体质量等),血液化验结果(肝肾功能、血常规等),影像学检查结果(肿瘤数目、血管包膜侵犯、肿瘤最大直径等)。入组患者均在术后通过住院资料及电话咨询等方式进行回顾性随访统计,咨询和统计内容包括临床查体,血液学检查(肿瘤标志物、血常规、肝肾功能及凝血功能等),影像学检查(胸部、腹部平扫及增强扫描CT或磁共振等)。总生存期(overall survival,OS)定义为:从患者接受治疗之日开始到患者死亡或最后一次临床随访的时间为止。最后一次临床回顾性随访(<5年)无复发则默认该患者5年时间内未复发。
1.4 伦理学审查
本研究方案经由川北医学院附属医院伦理委员会审批,符合医学伦理学规定。
1.5 统计学方法
采用SPSS 26.0软件对研究数据进行统计学分析。符合正态分布的计量资料以x±s表示,两组间比较采用t检验; 非正态分布的计量资料以M(P25~P75)表示,两组间比较采用Mann-Whitney U检验。计数资料两组间比较采用χ2检验或Fisher精确法检验。将差异具有统计学意义的指标纳入二元logistic回归分析。采用受试者工作特征(ROC)曲线下面积(AUC)评估其诊断效能。P<0.05为差异有统计学意义。
2. 结果
2.1 一般资料
共纳入232例BCLC 0/A期HCC患者,依据5年内复发状态分为复发组(103例)和非复发组(129例)。在所有纳入研究的根治性切除的BCLC 0/A期HCC患者中,复发部位绝大部分(83.4%)为肝内复发,其次为肺、腹腔,也包括骨、脑、淋巴结等。两组比较,BCLC 0/A分期差异无统计学意义(P>0.05)。其中性别、年龄、体质量、术前RBC、WBC、PLT、TBil、DBil、肿瘤数、分化程度、并发症、住院时间差异均无统计学意义(P值均>0.05);而术前AST、ALT复发组明显高于非复发组,术前Alb复发组明显低于非复发组(P值均<0.05);复发组HBsAg阳性、包膜侵犯、微血管侵犯(MVI)、肿瘤直径≥5 cm、肝硬化(中重度)、非R0切除、5年死亡数所占比例显著高于非复发组(P值均<0.05);复发组的OS明显低于非复发组(P<0.05)(表 1)。
表 1 术后5年内复发组与非复发组相关基线资料、并发症及结局的比较项目 复发组(n=103) 非复发组(n=129) 统计值 P值 住院时间(d) 9.84±3.98 10.23±4.72 t=-0.666 0.506 年龄(岁) 50.36±12.05 52.28±12.99 t=-1.155 0.249 体质量(kg) 56.94±4.85 56.68±6.70 t=0.337 0.745 OS(月) 18.0(8.0~31.0) 25.0(15.5~40.5) Z=-2.880 0.004 术前RBC(109/L) 4.63±0.68 4.53±0.59 t=1.166 0.245 术前WBC(109/L) 5.57±1.63 5.60±2.02 t=-0.438 0.662 术前PLT(109/L) 144(101~203) 138(92~200) Z=0.445 0.656 术前TBil(μmol/L) 14.7(11.7~19.3) 13.6(10.1~18.0) Z=1.666 0.096 术前DBil(μmol/L) 4.8(3.5~6.4) 4.5(3.6~5.8) Z=0.975 0.330 术前ALT(U/L) 51(39~63) 39(30~52) Z=4.587 <0.001 术前AST(U/L) 44(33~65) 34(25~51) Z=3.864 <0.001 术前Alb(g/L) 39.08±4.19 42.34±4.53 t=-5.628 <0.001 男性[例(%)] 84(81.6) 102(79.1) χ2=0.222 0.637 HBsAg阳性[例(%)] 87(84.5) 60(46.5) χ2=35.539 <0.001 包膜侵犯[例(%)] 48(46.6) 23(17.8) χ2=22.325 <0.001 肿瘤直径≥5 cm[例(%)] 86(83.5) 88(68.2) χ2=7.130 0.008 MVI[例(%)] 32(31.1) 15(11.6) χ2=13.398 <0.001 R0切除[例(%)] 79(76.7) 112(86.8) χ2=4.034 0.045 低分化程度[例(%)] 40(38.8) 53(41.1) χ2=0.121 0.728 肿瘤数≥2个[例(%)] 11(10.7) 15(11.6) χ2=0.052 0.820 5年死亡数[例(%)] 51(49.5) 29(22.5) χ2=18.527 <0.001 肝硬化(中重度)[例(%)] 34(33.0) 27(20.9) χ2=4.312 0.038 BCLC分期[例(%)] 0期 9(8.7) 8(6.2) χ2=0.543 0.461 A期 94(91.3) 121(93.8) 并发症[例(%)] 肺部感染 8(7.8) 8(6.2) χ2=0.219 0.640 腹腔感染 3(2.9) 2(1.6) 0.841 腹腔出血 2(1.9) 7(5.4) 0.172 腹腔积液 81(78.6) 88(68.2) χ2=3.146 0.076 胸腔积液 9(8.7) 5(3.9) χ2=2.387 0.122 复发部位[例(%)] 肝 86(83.5) 肺 5(4.9) 肝肺 3(2.9) 肝脑 1(1.0) 腹腔 5(4.9) 骨 1(1.0) 淋巴结 2(1.9) 2.2 影响BCLC 0/A期HCC患者术后5年复发的危险因素
以入组患者的基线资料及术前部分化验指标为自变量,术后5年内复发状态为因变量,进行二元logistic回归分析。单因素分析结果显示,术前Alb<40 g/L、术前ALT≥40 U/L、MVI、HBsAg阳性、包膜侵犯、肿瘤直径≥5 cm、肝硬化(中重度)、非R0切除均是根治性切除的BCLC 0/A期HCC患者术后5年复发的影响因素(P值均<0.05)(表 2)。
表 2 BCLC 0/A期HCC患者行根治性切除术5年复发的logistic单因素分析项目 B值 SE Wald P值 OR(95%CI) 术前Alb(<40 g/L) 1.563 0.286 29.804 <0.001 4.774(2.724~8.368) 术前ALT(≥40 U/L) 1.045 0.278 14.093 <0.001 2.843(1.648~4.906) MVI 1.231 0.348 12.551 <0.001 3.425(1.733~6.769) HBsAg阳性 1.833 0.324 31.955 <0.001 6.253(3.312~11.806) 肿瘤直径(≥5 cm) 0.857 0.326 6.921 0.009 2.357(1.244~4.464) 包膜侵犯 1.392 0.303 21.073 <0.001 4.022(2.220~7.287) 肝硬化(中重度) 0.621 0.301 4.255 0.039 1.862(1.031~3.360) 非R0切除 0.694 0.349 3.944 0.047 2.001(1.009~3.970) 将单因素分析有统计学意义的影响因素纳入多因素分析,结果显示,术前Alb<40 g/L(OR=5.796,P<0.001)、术前ALT≥40 U/L(OR=3.029,P=0.002)、MVI(OR=3.981,P=0.003)、HBsAg阳性(OR=7.829,P<0.001)、包膜侵犯(OR=5.357,P<0.001)、非R0切除(OR=3.048,P=0.018)均为根治性切除的BCLC 0/A期HCC患者术后5年复发的独立危险因素(表 3)。因此,对肝功能异常患者,术前应积极保肝、纠正低蛋白血症等对症治疗,对于HBsAg阳性、包膜侵犯、MVI、肿瘤直径≥5 cm、中重度肝硬化、非R0切除的患者告知复发风险,规律按时复诊十分关键。因此,本研究希望通过对危险因素进行联合分析,建立患者术后复发的预警系统。
表 3 BCLC 0/A期HCC患者行根治性切除术5年复发的logistic多因素分析项目 B值 SE Wald P值 OR(95%CI) 常量 -4.598 0.661 48.371 <0.001 0.010 术前Alb(<40 g/L) 1.757 0.376 21.826 <0.001 5.796(2.773~12.113) 术前ALT(≥40 U/L) 1.108 0.366 9.175 0.002 3.029(1.479~6.204) MVI 1.382 0.463 8.922 0.003 3.981(1.608~9.856) HBsAg阳性 2.058 0.413 24.884 <0.001 7.829(3.488~17.574) 肿瘤直径(≥5 cm) 0.606 0.410 2.187 0.139 1.833(0.821~4.091) 包膜侵犯 1.678 0.411 16.668 <0.001 5.357(2.393~11.992) 肝硬化(中重度) 0.406 0.406 1.001 0.317 1.502(0.677~3.329) 非R0切除 1.115 0.473 5.552 0.018 3.048(1.206~7.704) 2.3 肝癌根治性切除术后复发评分预警系统(PLC-EWSPRS)评分分布及差异
根据危险因素建立PLC-EWSPRS评分系统,其中独立危险因素赋值为2分,危险因素赋值为1分,该评分系统的阈值为0~14分,统计分析结果显示复发组最低分2分,最高分14分,平均(8.39±2.58)分; 非复发组最低分0分,最高分11分,平均(4.33±2.35)分; 即复发组的最低分与最高分均高于非复发组。将其分为0~5、6~10、11~14低中高3个分数段。复发组病例主要分布在中、高分数段,非复发组病例主要分布在低、中分数段,复发组中高分数段所占比例远高于非复发组,即中高分数段的复发率显著高于低分段(P<0.05)(表 4)。
表 4 两组患者PLC-EWSPRS评分的分布及复发差异分析分值分段(复发率) 分值 各分值病例分布[例(%)] 分值段病例分布[例(%)] χ2值 P值 复发组(n=103) 非复发组(n=129) 复发组(n=103) 非复发组(n=129) 低分段(0~5) 0 0 5(3.9) 91.502 <0.001 (9.8%) 1 0 15(11.6) 2 2(1.9) 8(6.2) 3 2(1.9) 18(14.0) 4 2(1.9) 18(14.0) 5 4(3.9) 28(21.7) 10(9.7) 92(71.3) 中分段(6~10) 6 14(13.6) 16(12.4) (68.2%) 7 10(9.7) 14(10.9) 8 25(24.3) 1(0.8) 9 12(11.7) 2(1.6) 10 14(14.6) 2(1.6) 75(72.8) 35(27.1) 高分段(11~14) 11 2(1.9) 2(1.6) (90.0%) 12 8(7.8) 0 13 6(5.8) 0 14 2(1.9) 0 18(17.5) 2(1.6) 2.4 诊断预测效能
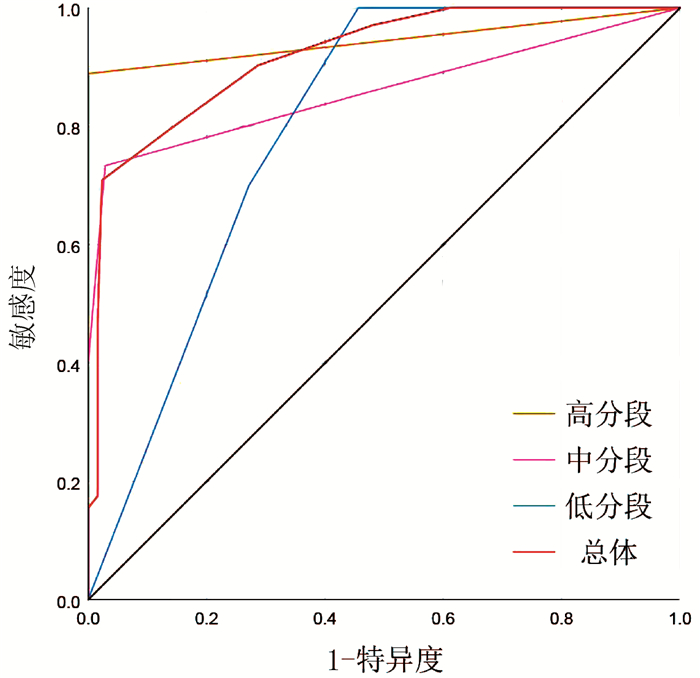
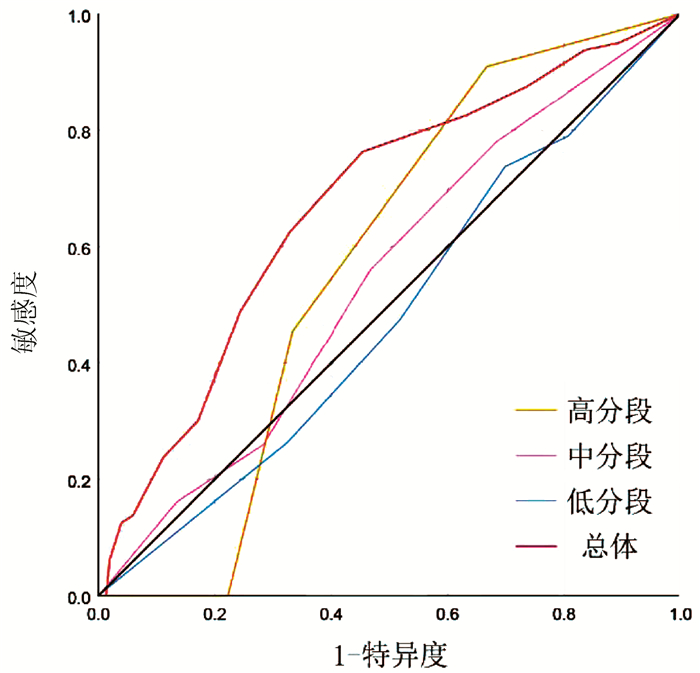
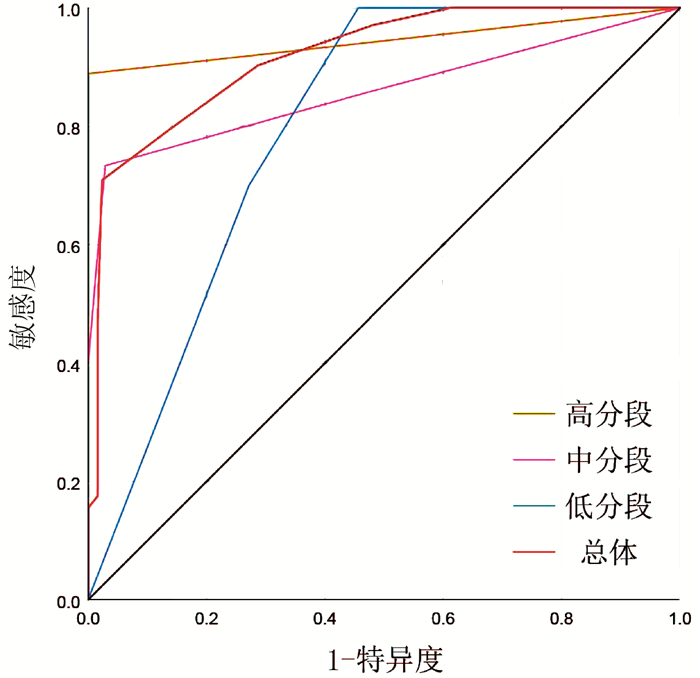
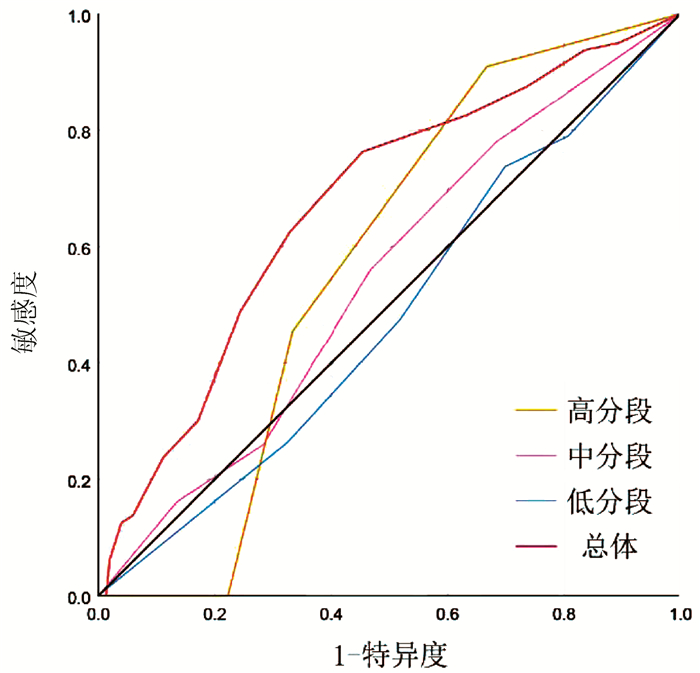
为了进一步证实PLC-EWSPRS评分系统评分越高,复发风险越大,具有复发风险预测价值,同时评估其对根治性切除的BCLC 0/A期HCC患者术后5年生存状态是否具有预测价值,本研究对该系统评分进行诊断效能分析。ROC曲线分析结果显示,预测根治性切除的BCLC 0/A期HCC患者术后5年死亡的AUC(95%CI)为0.672(0.599~0.744),P<0.001; 亚组预测低、中、高分数段术后5年死亡的AUC(95%CI)分别为:0.478(0.338~0.619), P=0.595;0.544(0.436~0.652), P=0.425;0.571(0.315~0.826), P=0.770。预测根治性切除的BCLC 0/A期HCC患者术后5年内复发的AUC(95%CI)为0.918(0.883~0.953), P<0.001;亚组预测低、中、高分数段术后5年内复发的AUC(95%CI)分别为:0.796(0.695~0.896), P=0.002;0.859(0.791~0.927), P<0.001;0.944(0.839~1.000), P=0.044。因此,PLC-EWSPRS系统对根治性切除的BCLC 0/A期HCC患者术后5年内生存状态预测价值有限,各亚组预测无意义(P>0.05);而对术后5年复发具有良好的预测价值,各亚组预测价值仍较高(P<0.05)(图 1、2)。
3. 讨论
PLC严重威胁着人类的健康,是全球癌症相关死亡的第三大主要原因[5],根治性外科切除是HCC极其重要的治疗方式[6]。HCC治疗方式的选择当前主要以BCLC分期为主要参考标准,其中BCLC 0/A期主要首选根治性手术[3]。虽然已经有研究预扩大BCLC肝癌切除标准,即对BCLC-B甚至BCLC-C期HCC患者也行手术切除治疗[7]。已经有部分回顾性分析提出肝切除术能够使部分BCLC B/C期患者取得可接受的结果[7-8],但还需要大量前瞻性临床研究加以证实。因此,本研究选择以BCLC 0/A期HCC患者为研究对象,探究术后复发的相关问题。早期HCC往往具有更大的手术治疗机会,掌握手术时机及积极术前准备均十分关键[9]。尽管BCLC 0/A期HCC多数具有良好的手术治疗指征,本应取得良好的手术效果,然而肝癌切除术后5年肿瘤复发转移率高达40%~70%[10],本研究术后5年复发率为44.40%,亦在此高发范围内。并且在本研究中复发组的5年病死率为49.5%,非复发组的5年病死率为22.5%,由此可见术后复发严重制约着肝癌患者术后生存。因此,建立一个全面系统的肝癌术后复发预警系统十分关键,通过预警系统为患者拟定个性化的治疗及复诊方案,以达到预防或减少复发、提高OS的目的。
HBV可促进中国人群肝癌的侵袭性[11]。抗病毒治疗应贯穿乙型肝炎相关HCC的外科手术前、术后及非手术治疗的全过程[12]。既往研究提出术前HBV DNA高负荷是肝癌术后复发的独立危险因素,术后复发预示着更低的复发后生存率[7],这与本研究证实HBsAg阳性为术后复发独立危险因素结果一致。Wu等[13]通过多因素分析表明HBV DNA水平>106拷贝/ml与远期复发(>2年)显著相关,因此术前降低病毒滴度和术后抗病毒及抗炎治疗对降低复发至关重要。有研究[14-15]表明肿瘤直径与早期复发及MVI发生也显著相关,而MVI与肝癌预后不良及早期复发亦密切相关[11, 16-17]。对肿瘤肌肉壁及距包膜1 cm外的血管被侵润的患者,术后更加容易复发[18]。但MVI目前只能通过术后病理获得,具有局限性,迫切需要新的指标和方法来预测MVI的发生[19]。也有更早的研究[20]提出肿瘤直径≥5 cm为肝切除术后1年内进展导致早期死亡的独立危险因素。以上都表明肿瘤直径及MVI与预后不良密切相关。对于肝功能异常的患者,如:低蛋白血症、转氨酶升高、高胆红素血症等,需要积极纠正后再行手术治疗。有学者[21]总结肝内胆管癌及HCC行手术治疗时,提出切缘阴性(R0切除)可显著降低复发、提高生存率,并且如果能够保留足够的功能性肝残余,建议对HCC和肝内胆管癌进行宽切缘的解剖切除,与本研究结果相同,也体现了行R0切除的必要性。要求术者经验丰富,做到精准解剖十分关键。包膜侵犯与HCC患者的总体生存及术后复发密切相关,对于HCC术后化疗患者,包膜浸润为OS的独立危险因素[22]。以上研究结果均与本研究确立的危险因素吻合,因此将这些危险因素纳入预测系统。
面对目前尚无针对术后复发有效预测系统的现状,PLC-EWSPRS评分系统的建立具有重要意义。并且该系统通过危险因素进行赋值评分加以汇总,综合临床指标更加全面具体,比单一因素具有更强的客观预测价值。在本研究中发现,复发组中高分数段所占比例远高于非复发组,提示该系统评分越高,复发风险越大,应该引起临床医师及患者的注意。根据总体及分层分析的ROC曲线的AUC值可知,PLC-EWSPRS系统对根治性切除的BCLC 0/A期HCC患者术后5年内生存状态预测价值有限,而对术后5年复发具有良好的预测价值。
本研究为单中心、小样本的回顾性研究,研究中两组患者肿瘤数和肿瘤分化程度无显著差异,与目前共识存在差异,未能纳入进行分析,这可能与研究样本量及病例纳入标准不同有关。另外,因目前肝癌术后复发相关预警系统罕见,未能进行预测价值的对比分析。后续还可以扩大纳入对象范围,以BCLC分期进行亚组分析,评估该系统对不同分期患者是否均具有术后复发预测价值。最后,因纳入对象的无瘤生存时间不明确病例较多,故未能行时间相关性的Cox回归分析,在后续验证中可以进行探究。因此,该系统还需要大样本、多中心、前瞻性的研究加以完善。但不能否认其在预测术后复发中所表现出的潜在价值。
综上,PLC-EWSPRS对BCLC 0/A期HCC患者术后5年复发具有良好的预测价值,对根治性切除的BCLC 0/A期HCC患者术后复查及治疗策略的制订具有重要的指导意义。
-
表 1 术后5年内复发组与非复发组相关基线资料、并发症及结局的比较
项目 复发组(n=103) 非复发组(n=129) 统计值 P值 住院时间(d) 9.84±3.98 10.23±4.72 t=-0.666 0.506 年龄(岁) 50.36±12.05 52.28±12.99 t=-1.155 0.249 体质量(kg) 56.94±4.85 56.68±6.70 t=0.337 0.745 OS(月) 18.0(8.0~31.0) 25.0(15.5~40.5) Z=-2.880 0.004 术前RBC(109/L) 4.63±0.68 4.53±0.59 t=1.166 0.245 术前WBC(109/L) 5.57±1.63 5.60±2.02 t=-0.438 0.662 术前PLT(109/L) 144(101~203) 138(92~200) Z=0.445 0.656 术前TBil(μmol/L) 14.7(11.7~19.3) 13.6(10.1~18.0) Z=1.666 0.096 术前DBil(μmol/L) 4.8(3.5~6.4) 4.5(3.6~5.8) Z=0.975 0.330 术前ALT(U/L) 51(39~63) 39(30~52) Z=4.587 <0.001 术前AST(U/L) 44(33~65) 34(25~51) Z=3.864 <0.001 术前Alb(g/L) 39.08±4.19 42.34±4.53 t=-5.628 <0.001 男性[例(%)] 84(81.6) 102(79.1) χ2=0.222 0.637 HBsAg阳性[例(%)] 87(84.5) 60(46.5) χ2=35.539 <0.001 包膜侵犯[例(%)] 48(46.6) 23(17.8) χ2=22.325 <0.001 肿瘤直径≥5 cm[例(%)] 86(83.5) 88(68.2) χ2=7.130 0.008 MVI[例(%)] 32(31.1) 15(11.6) χ2=13.398 <0.001 R0切除[例(%)] 79(76.7) 112(86.8) χ2=4.034 0.045 低分化程度[例(%)] 40(38.8) 53(41.1) χ2=0.121 0.728 肿瘤数≥2个[例(%)] 11(10.7) 15(11.6) χ2=0.052 0.820 5年死亡数[例(%)] 51(49.5) 29(22.5) χ2=18.527 <0.001 肝硬化(中重度)[例(%)] 34(33.0) 27(20.9) χ2=4.312 0.038 BCLC分期[例(%)] 0期 9(8.7) 8(6.2) χ2=0.543 0.461 A期 94(91.3) 121(93.8) 并发症[例(%)] 肺部感染 8(7.8) 8(6.2) χ2=0.219 0.640 腹腔感染 3(2.9) 2(1.6) 0.841 腹腔出血 2(1.9) 7(5.4) 0.172 腹腔积液 81(78.6) 88(68.2) χ2=3.146 0.076 胸腔积液 9(8.7) 5(3.9) χ2=2.387 0.122 复发部位[例(%)] 肝 86(83.5) 肺 5(4.9) 肝肺 3(2.9) 肝脑 1(1.0) 腹腔 5(4.9) 骨 1(1.0) 淋巴结 2(1.9) 表 2 BCLC 0/A期HCC患者行根治性切除术5年复发的logistic单因素分析
项目 B值 SE Wald P值 OR(95%CI) 术前Alb(<40 g/L) 1.563 0.286 29.804 <0.001 4.774(2.724~8.368) 术前ALT(≥40 U/L) 1.045 0.278 14.093 <0.001 2.843(1.648~4.906) MVI 1.231 0.348 12.551 <0.001 3.425(1.733~6.769) HBsAg阳性 1.833 0.324 31.955 <0.001 6.253(3.312~11.806) 肿瘤直径(≥5 cm) 0.857 0.326 6.921 0.009 2.357(1.244~4.464) 包膜侵犯 1.392 0.303 21.073 <0.001 4.022(2.220~7.287) 肝硬化(中重度) 0.621 0.301 4.255 0.039 1.862(1.031~3.360) 非R0切除 0.694 0.349 3.944 0.047 2.001(1.009~3.970) 表 3 BCLC 0/A期HCC患者行根治性切除术5年复发的logistic多因素分析
项目 B值 SE Wald P值 OR(95%CI) 常量 -4.598 0.661 48.371 <0.001 0.010 术前Alb(<40 g/L) 1.757 0.376 21.826 <0.001 5.796(2.773~12.113) 术前ALT(≥40 U/L) 1.108 0.366 9.175 0.002 3.029(1.479~6.204) MVI 1.382 0.463 8.922 0.003 3.981(1.608~9.856) HBsAg阳性 2.058 0.413 24.884 <0.001 7.829(3.488~17.574) 肿瘤直径(≥5 cm) 0.606 0.410 2.187 0.139 1.833(0.821~4.091) 包膜侵犯 1.678 0.411 16.668 <0.001 5.357(2.393~11.992) 肝硬化(中重度) 0.406 0.406 1.001 0.317 1.502(0.677~3.329) 非R0切除 1.115 0.473 5.552 0.018 3.048(1.206~7.704) 表 4 两组患者PLC-EWSPRS评分的分布及复发差异分析
分值分段(复发率) 分值 各分值病例分布[例(%)] 分值段病例分布[例(%)] χ2值 P值 复发组(n=103) 非复发组(n=129) 复发组(n=103) 非复发组(n=129) 低分段(0~5) 0 0 5(3.9) 91.502 <0.001 (9.8%) 1 0 15(11.6) 2 2(1.9) 8(6.2) 3 2(1.9) 18(14.0) 4 2(1.9) 18(14.0) 5 4(3.9) 28(21.7) 10(9.7) 92(71.3) 中分段(6~10) 6 14(13.6) 16(12.4) (68.2%) 7 10(9.7) 14(10.9) 8 25(24.3) 1(0.8) 9 12(11.7) 2(1.6) 10 14(14.6) 2(1.6) 75(72.8) 35(27.1) 高分段(11~14) 11 2(1.9) 2(1.6) (90.0%) 12 8(7.8) 0 13 6(5.8) 0 14 2(1.9) 0 18(17.5) 2(1.6) -
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. [2] European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis[J]. J Hepatol, 2018, 69(2): 406-460. DOI: 10.1016/j.jhep.2018.03.024. [3] TSILIMIGRAS DI, BAGANTE F, SAHARA K, et al. Prognosis after resection of Barcelona Clinic Liver Cancer (BCLC) stage 0, A, and B hepatocellular carcinoma: A comprehensive assessment of the current BCLC classification[J]. Ann Surg Oncol, 2019, 26(11): 3693-3700. DOI: 10.1245/s10434-019-07580-9. [4] FORNER A, LLOVET JM, BRUIX J. Hepatocellular carcinoma[J]. Lancet, 2012, 379(9822): 1245-1255. DOI: 10.1016/S0140-6736(11)61347-0. [5] YANG WS, ZENG XF, LIU ZN, et al. Diet and liver cancer risk: A narrative review of epidemiological evidence[J]. Br J Nutr, 2020, 124(3): 330-340. DOI: 10.1017/S0007114520001208. [6] XU W, LIU F, SHEN X, et al. Prognostic nomograms for patients with hepatocellular carcinoma after curative hepatectomy, with a focus on recurrence timing and post-recurrence management[J]. J Hepatocell Carcinoma, 2020, 7: 233-256. DOI: 10.2147/JHC.S271498. [7] LIU YW, YONG CC, LIN CC, et al. Liver resection of hepatocellular carcinoma within and beyond the Barcelona Clinic Liver Cancer guideline recommendations: Results from a high-volume liver surgery center in East Asia[J]. J Surg Oncol, 2020, 122(8): 1587-1594. DOI: 10.1002/jso.26183. [8] TSILIMIGRAS DI, BAGANTE F, MORIS D, et al. Recurrence patterns and outcomes after resection of hepatocellular carcinoma within and beyond the barcelona clinic liver cancer criteria[J]. Ann Surg Oncol, 2020, 27(7): 2321-2331. DOI: 10.1245/s10434-020-08452-3. [9] KABIR T, SYN N, RAMKUMAR M, et al. Effect of surgical delay on survival outcomes in patients undergoing curative resection for primary hepatocellular carcinoma: Inverse probability of treatment weighting using propensity scores and propensity score adjustment[J]. Surgery, 2020, 167(2): 417-424. DOI: 10.1016/j.surg.2019.09.022. [10] Medical Administration of the National Health Commission of the people's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007.中华人民共和国国家卫生健康委员会政医管局. 原发性肝癌诊疗规范(2019年版)[J]. 临床肝胆病杂志, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. [11] YANG F, MA L, YANG Y, et al. Contribution of hepatitis B virus infection to the aggressiveness of primary liver cancer: A clinical epidemiological study in Eastern China[J]. Front Oncol, 2019, 9: 370. DOI: 10.3389/fonc.2019.00370. [12] WANG S, WU QW, LI XK, et al. Interpretation of guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(5): 996-999. DOI: 10.3969/j.issn.1001-5256.2020.05.009.王姗, 吴庆旺, 李小科, 等. 《原发性肝癌诊疗规范(2019年版)》解读[J]. 临床肝胆病杂志, 2020, 36(5): 996-999. DOI: 10.3969/j.issn.1001-5256.2020.05.009. [13] WU JC, HUANG YH, CHAU GY, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma[J]. J Hepatol, 2009, 51(5): 890-897. DOI: 10.1016/j.jhep.2009.07.009. [14] WANG B, XIA CY, LAU WY, et al. Determination of clonal origin of recurrent hepatocellular carcinoma for personalized therapy and outcomes evaluation: A new strategy for hepatic surgery[J]. J Am Coll Surg, 2013, 217(6): 1054-1062. DOI: 10.1016/j.jamcollsurg.2013.07.402. [15] KIM JM, KWON CH, JOH JW, et al. Intrahepatic metastasis is more risky than multiple occurrence in hepatocellular carcinoma patients after curative liver resection[J]. Hepatogastroenterology, 2015, 62(138): 399-404. DOI: 10.5754/hge131006. [16] LIN S, YE F, RONG W, et al. Nomogram to assist in surgical plan for hepatocellular carcinoma: A prediction model for microvascular invasion[J]. J Gastrointest Surg, 2019, 23(12): 2372-2382. DOI: 10.1007/s11605-019-04140-0. [17] ZHANG X, LI J, SHEN F, et al. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma[J]. J Gastroenterol Hepatol, 2018, 33(2): 347-354. DOI: 10.1111/jgh.13843. [18] ROAYAIE S, BLUME IN, THUNG SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma[J]. Gastroenterology, 2009, 137(3): 850-855. DOI: 10.1053/j.gastro.2009.06.003. [19] ERSTAD DJ, TANABE KK. Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma[J]. Ann Surg Oncol, 2019, 26(5): 1474-1493. DOI: 10.1245/s10434-019-07227-9. [20] REGIMBEAU JM, ABDALLA EK, VAUTHEY JN, et al. Risk factors for early death due to recurrence after liver resection for hepatocellular carcinoma: Results of a multicenter study[J]. J Surg Oncol, 2004, 85(1): 36-41. DOI: 10.1002/jso.10284. [21] LAFARO K, GRANDHI MS, HERMAN JM, et al. The importance of surgical margins in primary malignancies of the liver[J]. J Surg Oncol, 2016, 113(3): 296-303. DOI: 10.1002/jso.24123. [22] HU W, HUANG S, DONG L, et al. MDR1 gene polymorphism correlated with pathological characteristics and prognosis in patients with primary hepatocellular carcinoma receiving interventional therapy[J]. Anticancer Drugs, 2019, 30(3): 233-240. DOI: 10.1097/CAD.0000000000000680. 期刊类型引用(1)
1. 王叮叮. MEWS联合qSOFA评分在肿瘤危重症患者中应用价值. 中国城乡企业卫生. 2022(09): 112-114 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 2961 KB)
PDF下载 ( 2961 KB)

 下载:
下载:


 下载:
下载:
 百度学术
百度学术