立体定向放疗序贯仑伐替尼治疗中晚期原发性肝癌的效果与安全性分析
DOI: 10.3969/j.issn.1001-5256.2021.09.023
利益冲突声明:本研究不存在研究者、伦理委员会成员、受试者监护人以及与公开研究成果有关的利益冲突。
作者贡献声明:纪晓权、孙静负责起草及撰写文章; 张爱民、张弢、李文刚、何卫平负责数据收集、统计与随访; 段学章负责修改文章并最终定稿。
Efficacy and safety of sequential lenvatinib therapy after stereotactic body radiotherapy in treatment of advanced primary liver cancer
-
摘要:
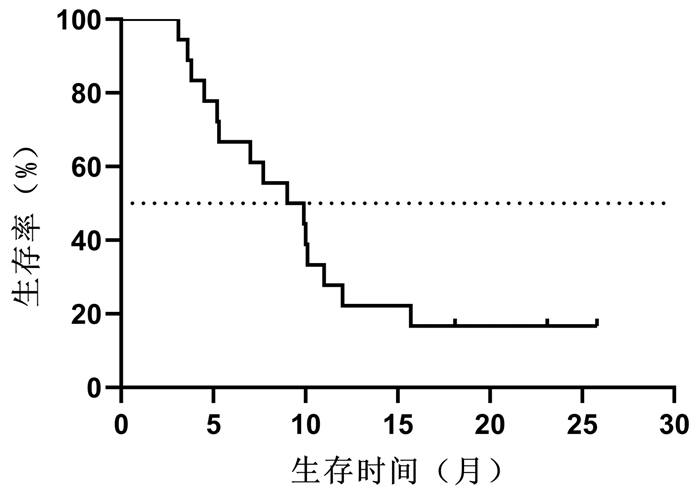
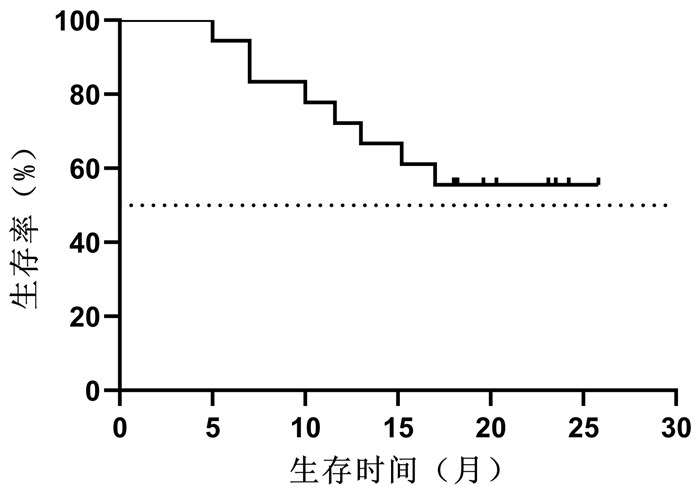
目的 探讨立体定向放射治疗(SBRT)序贯仑伐替尼治疗中晚期原发性肝癌的效果与安全性。 方法 选取2018年10月—2019年5月解放军总医院第五医学中心收治的18例中晚期原发性肝癌行SBRT联合仑伐替尼治疗患者。其中,BCLC B期4例,C期14例。给予计划靶区处方剂量48~55 Gy,中位值50 Gy,分6~10次,单次剂量中位值6(5~9)Gy/次。完成SBRT 1周后开始口服仑伐替尼治疗,用药中位时间为9.5(3.6~25.8)个月。治疗后前3个月,每月随访1次。治疗3个月后,每3个月随访1次。采用Kaplan-Meier法计算总生存、无进展生存、局部控制率,观察不良反应和并发症发生率。 结果 随访至2020年11月30日,共有8例患者死亡,其中3例因肝衰竭死亡,3例因肿瘤进展死亡,1例因胆囊穿孔死亡,1例因消化道出血死亡。治疗开始后3、6、9、12、18个月的总生存率分别为100%、94%、83%、72%、67%,无进展生存率分别为100%、67%、50%、22%、17%,局部控制率为100%、94%、94%、94%、94%,其中中位总生存期>18个月,中位无进展生存期为9个月。1例(6%)患者SBRT过程中出现3级不良反应,2例(11%)患者仑伐替尼治疗过程中出现3级不良反应,未发生致命性不良反应。 结论 初步证实SBRT序贯仑伐替尼是中晚期原发性肝癌有效、安全的治疗方法。 Abstract:Objective To investigate the efficacy and safety of sequential lenvatinib therapy after stereotactic body radiotherapy (SBRT) in the treatment of advanced primary liver cancer. Methods A total of 18 patients with advanced primary liver cancer who were admitted to The Fifth Medical Center of Chinese PLA General Hospital from October 2018 to May 2019 were enrolled, among whom there were 4 patients with BCLC stage B liver cancer and 14 patients with BCLC stage C liver cancer. The prescribed dose of planning target volume was 48-55 Gy (median 50 Gy) in 6-9 fractions, and the median of single dose was 6 (5-9) Gy per fraction. Oral administration of lenvatinib was given since 1 week after SBRT was finished, with a median medication time of 9.5 (3.6-25.8) months. Follow-up was performed once a month for the first 3 months after treatment and once every 3 months after 3 months of treatment. The Kaplan-Meier method was used to calculate overall survival (OS) rate, progression-free survival (PFS) rate, and local control (LC) rate, and the incidence rates of adverse reactions and complications were also observed. Results Up to the follow-up on November 30, 2020, a total of 8 patients died, among whom 3 died of liver failure, 3 died due to tumor progression, 1 died of perforation of gallbladder, and 1 died of gastrointestinal bleeding. At 3, 6, 9, 12, and 18 months of treatment, the OS rates were 100%, 94%, 83%, 72%, and 67%, respectively, the PFS rates were 100%, 67%, 50%, 22%, and 17%, respectively, and the LC rates were 100%, 94%, 94%, 94%, and 94%, respectively; the median OS time was > 18 months, and the median PFS time was 9 months. Of all patients, 1 (6%) had a grade 3 adverse reaction during SBRT and 2 (11%) experienced a grade 3 adverse reaction during lenvatinib treatment, and no fatal adverse reaction was observed. Conclusion It is preliminarily proved that sequential lenvatinib therapy after SBRT is an effective and safe treatment method for advanced primary liver cancer. -
Key words:
- Liver Neoplasms /
- Radiosurgery /
- Lenvatinib /
- Treatment Outcome
-
表 1 SBRT和仑伐替尼治疗不良反应
项目 SBRT[例(%)] 服用仑伐替尼[例(%)] 1~2级 3级 4~5级 1~2级 3级 4~5级 纳差 3(17) 0 0 0 0 0 恶心 3(17) 1(6) 0 0 0 0 呕吐 0 1(6) 0 0 0 0 高血压 0 0 0 6(33) 0 0 蛋白尿 0 0 0 3(17) 0 0 腹痛 0 0 0 2(11) 1(6) 0 腹泻 0 0 0 1(6) 1(6) 0 体质量下降 0 0 0 2(11) 0 0 疲乏 0 0 0 2(11) 0 0 腹胀 0 0 0 1(6) 0 0 -
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. [2] ZHENG RS, SUN KX, ZHANG SW, et al. Report of cancer epidemiology in China, 2015[J]. Chin J Oncol, 2019, 41(1): 19-28. DOI: 10.3760/cma.j.issn.0253-3766.2019.01.008.郑荣寿, 孙可欣, 张思维, 等. 2015年中国恶性肿瘤流行情况分析[J]. 中华肿瘤杂志, 2019, 41(1): 19-28. DOI: 10.3760/cma.j.issn.0253-3766.2019.01.008. [3] BRUIX J, SHERMAN M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update[J]. Hepatology, 2011, 53(3): 1020-1022. DOI: 10.1002/hep.24199. [4] LAU WY, LAI EC. Salvage surgery following downstaging of unresectable hepatocellular carcinoma—a strategy to increase resectability[J]. Ann Surg Oncol, 2007, 14(12): 3301-3309. DOI: 10.1245/s10434-007-9549-7. [5] LLOVET JM, BRÚ C, BRUIX J. Prognosis of hepatocellular carcinoma: The BCLC staging classification[J]. Semin Liver Dis, 1999, 19(3): 329-338. DOI: 10.1055/s-2007-1007122. [6] Bureau of Medical Administration, National Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007.中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗规范(2019年版)[J]. 临床肝胆病杂志, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. [7] TSE RV, HAWKINS M, LOCKWOOD G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma[J]. J Clin Oncol, 2008, 26(4): 657-664. DOI: 10.1200/JCO.2007.14.3529. [8] STERZING F, BRUNNER TB, ERNST I, et al. Stereotactic body radiotherapy for liver tumors: Principles and practical guidelines of the DEGRO Working Group on Stereotactic Radiotherapy[J]. Strahlenther Onkol, 2014, 190(10): 872-881. DOI: 10.1007/s00066-014-0714-1. [9] MÉNDEZ ROMERO A, WUNDERINK W, van OS RM, et al. Quality of life after stereotactic body radiation therapy for primary and metastatic liver tumors[J]. Int J Radiat Oncol Biol Phys, 2008, 70(5): 1447-1452. DOI: 10.1016/j.ijrobp.2007.08.058. [10] MA S, JIAO B, LIU X, et al. Approach to radiation therapy in hepatocellular carcinoma[J]. Cancer Treat Rev, 2010, 36(2): 157-163. DOI: 10.1016/j.ctrv.2009.11.008. [11] ANDOLINO DL, JOHNSON CS, MALUCCIO M, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma[J]. Int J Radiat Oncol Biol Phys, 2011, 81(4): e447-e453. DOI: 10.1016/j.ijrobp.2011.04.011. [12] BENEDICT SH, YENICE KM, FOLLOWILL D, et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101[J]. Med Phys, 2010, 37(8): 4078-4101. DOI: 10.1118/1.3438081. [13] KUDO M, FINN RS, QIN S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial[J]. Lancet, 2018, 391(10126): 1163-1173. DOI: 10.1016/S0140-6736(18)30207-1. [14] CHEN AP, SETSER A, ANADKAT MJ, et al. Grading dermatologic adverse events of cancer treatments: The common terminology criteria for adverse events version 4.0[J]. J Am Acad Dermatol, 2012, 67(5): 1025-1039. DOI: 10.1016/j.jaad.2012.02.010. [15] PAN CC, KAVANAGH BD, DAWSON LA, et al. Radiation-associated liver injury[J]. Int J Radiat Oncol Biol Phys, 2010, 76(3 Suppl): S94-S100. DOI: 10.1016/j.ijrobp.2009.06.092. [16] LENCIONI R, LLOVET JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma[J]. Semin Liver Dis, 2010, 30(1): 52-60. DOI: 10.1055/s-0030-1247132. [17] SHAH C, MRAMBA LK, BISHNOI R, et al. Survival differences among patients with hepatocellular carcinoma based on the stage of disease and therapy received: Pre and post sorafenib era[J]. J Gastrointest Oncol, 2017, 8(5): 789-798. DOI: 10.21037/jgo.2017.06.16. [18] RIM CH, KIM CY, YANG DS, et al. Comparison of radiation therapy modalities for hepatocellular carcinoma with portal vein thrombosis: A meta-analysis and systematic review[J]. Radiother Oncol, 2018, 129(1): 112-122. DOI: 10.1016/j.radonc.2017.11.013. [19] SUN J, ZHANG AM, LI WG, et al. Observation of clinical efficacy of stereotactic body radiotherapy in 28 cases of large hepatocellular carcinoma[J]. Clin J Radiat Oncol, 2019, 28(10): 749-752. DOI: 10.3760/cma.j.issn.1004-4221.2019.10.007.孙静, 张爱民, 李文刚, 等. 28例大肝癌立体定向放疗疗效观察[J]. 中华放射肿瘤学杂志, 2019, 28(10): 749-752. DOI: 10.3760/cma.j.issn.1004-4221.2019.10.007. [20] BRADE AM, NG S, BRIERLEY J, et al. Phase 1 trial of sorafenib and stereotactic body radiation therapy for hepatocellular carcinoma[J]. Int J Radiat Oncol Biol Phys, 2016, 94(3): 580-587. DOI: 10.1016/j.ijrobp.2015.11.048. [21] FINN RS, QIN S, IKEDA M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382(20): 1894-1905. DOI: 10.1056/NEJMoa1915745. -



 PDF下载 ( 2112 KB)
PDF下载 ( 2112 KB)

 下载:
下载: