Therapeutic effect of Taohong Siwu decoction on a mouse model of carbon tetrachloride-induced liver fibrosis and its mechanism
-
摘要:
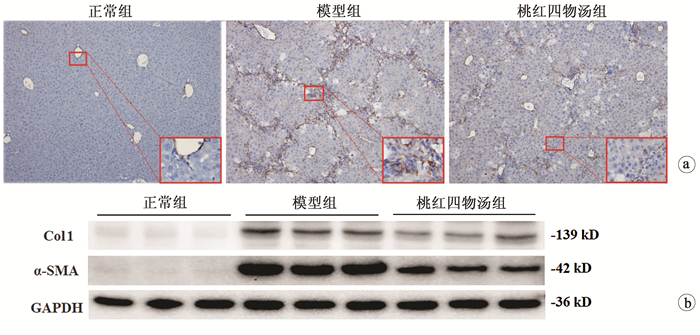
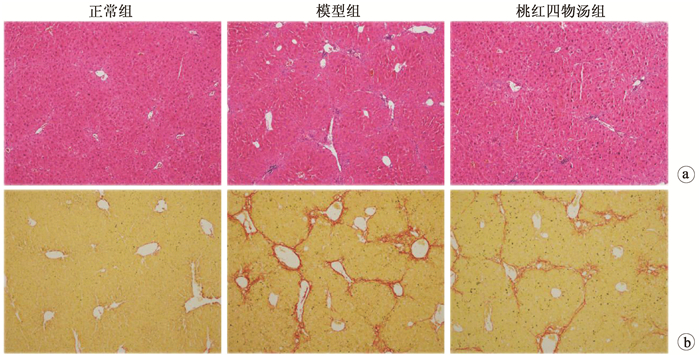
目的 探讨桃红四物汤对CCl4诱导肝纤维化小鼠模型的干预作用及其作用机制。 方法 24只雄性C57/BL6小鼠随机分为对照组、模型组和桃红四物汤组,每组8只。模型组和桃红四物汤组小鼠腹腔注射10%CCl4,第3周开始给予桃红四物汤灌胃给药4周,检测肝功能(ALT、AST),观测肝组织病理形态学。实时荧光定量PCR检测α-平滑肌肌动蛋白(α-SMA)、透明质酸合成酶-2(HAS-2)、Ⅰ型胶原(Col1)mRNA的表达,蛋白免疫印迹法检测α-SMA、Col1及HAS-2蛋白的表达。分离小鼠原代肝星状细胞(HSC),应用siRNA沉默HAS-2,检测其对HSC激活的影响。计量资料两组间比较采用t检验,多组间比较采用单因素方差分析,组内进一步两两比较采用SNK或LSD-t检验。 结果 与正常组相比,模型组血清肝功能指标ALT、AST水平显著升高,桃红四物汤组小鼠血清ALT、AST水平均显著下降(P值均<0.01)。病理学染色显示:模型组炎症细胞浸润明显,增生的胶原纤维形成纤维间隔;桃红四物汤组胶原纤维间隔较疏松及炎症细胞浸润减轻。与模型组相比,桃红四物汤组α-SMA、Col1 mRNA和蛋白的表达水平均显著降低(P值均<0.001)。与正常组比较,模型组肝组织中HAS-2 mRNA表达水平显著升高(t=6.14,P<0.05),桃红四物汤组HAS-2蛋白表达水平(0.29±0.10)较模型组(1.00±0.12)明显减少(t=70.73,P<0.001)。siRNA沉默HAS-2,TGFβ处理后(Si HAS-2+TGFβ组)α-SMA、Col1 mRNA表达水平较NC+TGFβ组显著降低(P<0.01)。 结论 桃红四物汤通过抑制HAS-2对CCl4致小鼠纤维化有显著治疗作用。 -
关键词:
- 肝硬化 /
- 桃红四物汤 /
- 透明质酸合成酶2 /
- 小鼠, 近交C57BL
Abstract:Objective To investigate the therapeutic effect of Taohong Siwu decoction on a mouse model of carbon tetrachloride (CCl4)-induced liver fibrosis and its mechanism of action. Methods A total of 24 male C57BL/6 mice were randomly divided into normal group, model group, and Taohong Siwu decoction group, with 8 mice in each group. The mice in the model group and the Taohong Siwu decoction group were given intraperitoneal injection of 10% CCl4, and Taohong Siwu decoction was given by gavage since week 3 for 4 consecutive weeks. Liver function [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)] was measured, and liver pathomorphology was observed. Real-time PCR was used to measure the mRNA expression of α-smooth muscle actin (α-SMA), hyaluronic acid synthase-2 (HAS-2), and collagen type Ⅰ(Col1), and Western blotting was used to measure the protein expression of α-SMA, Col1, and HAS-2. Primary hepatic stellate cells (HSCs) were isolated, and HAS-2 was silenced by siRNA to observe its influence on HSC activation. The t-test was used for comparison of continuous data between two groups; a one-way analysis of variance was used for comparison between multiple groups, and the SNK or least significant difference t-test was used for further comparison between two groups. Results Compared with the normal group, the model group had significant increases in serum liver function parameters (ALT, AST) and the Taohong Siwu decoction group had significant reductions in the serum levels of ALT and AST (all P < 0.01). Pathological staining showed that the model group had marked inflammatory cell infiltration and formation of fibrous septa by proliferated collagen fibers, and the Taohong Siwu decoction group had loose fibrous septa and alleviated inflammatory cell infiltration. Compared with the model group, the Taohong Siwu decoction group had significant reductions in the mRNA and protein expression of α-SMA and Col1(all P < 0.001). Compared with the normal group, the model group had a significant increase in the mRNA expression level of HAS-2 in liver tissue (t=6.14, P < 0.05), and compared with the model group, the Taohong Siwu decoction group had a significant reduction in the protein expression level of HAS-2 (0.29±0.10 vs 1.00±0.12, t=70.73, P < 0.001). After HAS-2 was silenced by siRNA, the Si HAS-2+transforming growth factor β (TGFβ) group (treated with TGFβ) had significant reductions in the mRNA expression levels of α-SMA and Col-Ⅰ compared with the NC+TGFβ group (P < 0.01). Conclusion Taohong Siwu decoction exerts a marked therapeutic effect on CCl4-induced liver fibrosis in mice by inhibiting HAS-2. -
表 1 RT-PCR引物序列
名称 序列 18S RNA 上游引物:5′-GTAACCCGTTGAACCCCATT-3′ 下游引物:5′-CCATCCAATCGGTAGTAGCG-3′ Col1 上游引物:5′-TAGGCCATTGTGTATGCAGC-3′ 下游引物:5′-ACATGTTCAGCTTTGTGGACC-3′ α-SMA 上游引物:5′-GTTCAGTGGTGCCTCTGTCA-3′ 下游引物:5′-ACTGGGACGACATGGAAAAG-3′ HAS-1 上游引物:5′-CTATGCTACCAAGTATACCTCG-3′ 下游引物:5′-TCTCGGAAGTAAGATTTGGAC-3′ HAS-2 上游引物:5′-TGAACAAAACGGTAGCAATCTG-3′ 下游引物:5′-ACTTTAATCCCAGGGTAGGTCAG-3′ HAS-3 上游引物:5′-GATGTCCAAATCCTCAACAAG-3′ 下游引物:5′-CCCACTAATACATTGCACAC-3′ 表 2 各组血清转氨酶水平及病理学改变
指标 正常组(n=8) 模型组(n=8) 桃红四物汤组(n=8) F值 P值 ALT(U/L) 6.61±1.92 247.33±21.761) 144.84±15.942) 10.75 <0.001 AST(U/L) 8.41±3.48 173.44±31.231) 108.05±18.452) 5.10 <0.001 炎性细胞面积(%) 2.76±0.98 12.37±1.091) 6.58±0.722) 12.52 <0.001 天狼星红面积(%) 1.20±0.35 10.80±2.491) 5.62±4.312) 5.50 <0.001 注:1)与正常组比较,P<0.01;2)与模型组比较,P<0.01。 表 3 各组α-SMA、Col1表达水平比较
指标 正常组(n=8) 模型组(n=8) 桃红四物汤组(n=8) F值 P值 免疫印迹 α-SMA/GADPH 1.00±0.12 10.79±0.471) 4.34±1.042) 169.96 <0.001 Col1/GADPH 1.00±0.54 7.56±0.961) 3.67±1.202) 36.98 <0.001 RT-PCR α-SMA mRNA 1.00±0.28 5.50±1.741) 3.12±0.702) 33.73 <0.001 Col1 mRNA 1.00±0.44 4.63±1.541) 2.98±1.292) 18.80 <0.001 注:1)与正常组比较,P<0.01;2)与模型组比较,P<0.01。 表 4 各组HAS-1、HAS-2、HAS-3mRNA表达情况
指标 正常组 模型组 t值 P值 HAS-1 1.00±0.19 1.22±0.49 1.46 0.247 HAS-2 1.00±0.04 2.59±0.17 6.14 0.027 HAS-3 1.00±0.24 0.97±0.31 0.00 0.962 表 5 α-SMA、Col1、HAS-2mRNA在原代HSC中的表达水平
指标 对照组 TGFβ处理组 t值 P值 α-SMA mRNA 1.00±0.53 50.59±6.28 -11.165 0.008 Col1 mRNA 1.00±0.63 30.39±1.49 -27.73 0.001 HAS-2 mRNA 1.00±0.47 9.72±0.64 -19.36 0.003 表 6 基因沉默HAS2检测THSWD对HSC活化的影响
组别 α-SMA mRNA Col1 mRNA NC 1.00±0.14 1.00±0.92 NC+TGFβ 52.98±4.891) 30.28±0.421) NC+TGFβ+THSWD 30.31±0.612) 18.33±0.902) Si HAS-2 0.90±0.077 1.53±0.24 Si HAS-2+TGFβ 25.11±2.512) 19.09±2.352) Si HAS-2+TGFβ+THSWD 24.44±1.05 20.68±2.08 F值 146.12 151.59 P值 <0.001 <0.001 注:NC,空白对照;1)与NC比较,P<0.01;2)与NC+TGFβ,比较P<0.01。 -
[1] PAROLA M, PINZANI M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues[J]. Mol Aspects Med, 2019, 65: 37-55. DOI: 10.1016/j.mam.2018.09.002. [2] LIU FW. The curative effect of Taohong Siwu Decoction in the treatment of decompensated cirrhosis and its effect on coagulation function[J]. Mod J Integr Tradit Chin West Med, 2016, 25(15): 1662-1664. DOI: 10.3969/j.issn.1008-8849.2016.15.023.刘发文. 桃红四物汤加减治疗失代偿期肝硬化的疗效及对凝血功能的影响[J]. 现代中西医结合杂志, 2016, 25(15): 1662-1664. DOI: 10.3969/j.issn.1008-8849.2016.15.023. [3] WANG WQ, WANG BX, SHAO WW. The effect of Angelica on the content of liver collagen protein of hepatic fibrosis rats induced by CCl4[J]. Heilongjiang Med Pharm, 2003, 26(3): 25. DOI: 10.3969/j.issn.1008-0104.2003.03.011.王伟群, 王柏欣, 邵文武. 当归对CCl4致大鼠肝纤维化胶原蛋白含量的影响[J]. 黑龙江医药科学, 2003, 26(3): 25. DOI: 10.3969/j.issn.1008-0104.2003.03.011. [4] WANG YQ. Anti hepatic fibrosis effect of peach kernel extract and its molecular mechanism[J]. J Med Theor & Pract, 2014, 27(15): 2015-2016. DOI:10.3969/j.issn.1008- 0104.2003.03.011.王玉清. 桃仁提取物抗肝纤维化作用及其分子机制研究[J]. 医学理论与实践, 2014, 27(15): 2015-2016. DOI:10.3969/j.issn.1008- 0104.2003.03.011. [5] MCQUITTY CE, WILLIAMS R, CHOKSHI S, et al. Immunomodulatory role of the extracellular matrix within the liver disease microenvironment[J]. Front Immunol, 2020, 11: 574276. DOI: 10.3389/fimmu.2020.574276. [6] WANG F, CHANG HM, YI Y, et al. TGF-β1 promotes hyaluronan synthesis by upregulating hyaluronan synthase 2 expression in human granulosa-lutein cells[J]. Cell Signal, 2019, 63: 109392. DOI: 10.1016/j.cellsig.2019.109392. [7] LIU C, YUAN X, TAO L, et al. Xia-yu-xue decoction (XYXD) reduces carbon tetrachloride (CCl4)-induced liver fibrosis through inhibition hepatic stellate cell activation by targeting NF-κB and TGF-β1 signaling pathways[J]. BMC Complement Altern Med, 2015, 15: 201. DOI: 10.1186/s12906-015-0733-1. [8] SHI K, LIU Y, WANG X, et al. Adjuvant Fuzheng Huayu Capsule reduces the incidence of hepatocellular carcinoma in patients with hepatitis B-caused cirrhosis[J]. Evid Based Complement Alternat Med, 2020, 2020: 8826091. DOI: 10.1155/2020/8826091. [9] ZHANG W, YANG GY, SHEN DX, et al. Mechanism of action of Xiayuxue decoction in inhibiting liver fibrosis by regulating glial cell line-derived neurotro phic factor[J]. J Clin Hepatol, 2021, 37(3): 575-581. DOI: 10.3969/j.issn.1001-5256.2021.03.015.张玮, 杨广越, 沈东晓, 等. 下瘀血汤抑制胶质细胞源性神经营养因子抗肝纤维化的作用机制[J]. 临床肝胆病杂志, 2021, 37 (3): 575-581. DOI: 10.3969/j.issn.1001-5256.2021.03.015. [10] LI ZY, TIAN YZ. Treatment of liver fibrosis by the Tibetan medicine Lang Qing A Ta according to stasis and heat[J]. J Changchun Univ Chin Med, 2019, 35(6): 1223-1225. DOI: 10.13463/j.cnki.cczyy.2019.06.061.李忠意, 田耀洲. 藏药郎庆阿塔从瘀热论治肝纤维化[J]. 长春中医药大学学报, 2019, 35(6): 1223-1225. DOI: 10.13463/j.cnki.cczyy.2019.06.061. [11] CHEN FY, TU CT. The role and molecular mechanism of Notch signaling transduction pathway on liver fibrogenesis[J/CD]. Chin J Liver Dis(Electronic Edition), 2020, 12(4): 23-28. DOI: 10.3969/j.issn.1674-7380.2020.04.004.陈方园, 涂传涛. Notch信号转导通路在肝纤维化形成中的作用与分子机制[J/CD]. 中国肝脏病杂志(电子版), 2020, 12(4): 23-28. DOI: 10.3969/j.issn.1674-7380.2020.04.004. [12] KISSELEVA T, BRENNER D. Molecular and cellular mechanisms of liver fibrosis and its regression[J]. Nat Rev Gastroenterol Hepatol, 2021, 18(3): 151-166. DOI: 10.1038/s41575-020-00372-7. [13] YUAN H, ZHAO C, BAO X, et al. Anti-liver fibrosis effect of total flavonoids from Scabiosa comosa Fisch. ex Roem. et Schult. on liver fibrosis in rat models and its proteomics analysis[J]. Ann Palliat Med, 2020, 9(2): 272-285. DOI: 10.21037/apm.2020.02.29. [14] PASSI A, VIGETTI D, BURASCHI S, et al. Dissecting the role of hyaluronan synthases in the tumor microenvironment[J]. FEBS J, 2019, 286(15): 2937-2949. DOI: 10.1111/febs.14847. [15] YANG YM, NOUREDDIN M, LIU C, et al. Hyaluronan synthase 2-mediated hyaluronan production mediates Notch1 activation and liver fibrosis[J]. Sci Transl Med, 2019, 11(496): eaatg284. DOI: 10.1126/scitranslmed.aat9284. -



 PDF下载 ( 4249 KB)
PDF下载 ( 4249 KB)

 下载:
下载: