Construction of a new patient-derived xenograft model of human liver cancer in mice with normal immunity
-
摘要:
目的 基于新型微载体microcarrier 6复合人原代肝癌细胞接种于具有正常免疫功能的小鼠,以期建立新型肝癌人源肿瘤异种移植(PDX)模型。 方法 从5例新鲜的人源肝癌组织中分离提取出原代肝癌细胞,分别与微载体microcarrier 6共培养,体外构建三维肿瘤细胞培养模型。将75只雄性C57BL/6小鼠按移植物不同随机分为3组:细胞对照组、微载体对照组和实验组(每例患者对应3组,共15组,每组5只)。采用皮下接种法将肝癌细胞-微载体复合体植入小鼠体内,观察小鼠成瘤时间、成瘤率、病理组织学表现等。计数资料两组间比较采用Fisher精确检验。 结果 5例患者的肝癌细胞接种小鼠后有3例对应的小鼠出现成瘤;此3例实验中,对照组均没有小鼠成瘤,仅实验组出现成瘤,实验组15只小鼠中有12只成功成瘤,成瘤率高达80%,与细胞对照组、微载体对照组相比差异均有统计学意义(P值均<0.05),其成瘤时间为5~7 d,移植瘤生长速度快,HE染色可见排列呈巢状或片状、异形性明显的细胞,可见病理性核分裂象,免疫组化染色CK8/18、Hep、Gpc-3均为阳性,符合人源肝癌细胞特点。 结论 本实验成功基于microcarrier 6复合人原代肝癌细胞在正常免疫小鼠体内建立了新型人源肝癌PDX模型,此模型可以更好地研究肝癌在免疫功能正常机体中发生、发展机制,同时也为肝癌精准医疗提供了较以往更有价值的新型动物模型。 Abstract:Objective To establish a new patient-derived xenograft (PDX) model of human liver cancer by inoculating the complex of human primary liver cancer cells and a novel microcarrier (microcarrier 6) into mice with normal immune function. Methods Primary liver cancer cells were isolated and extracted from the fresh human liver cancer tissue of five patients and were then co-cultured with microcarrier 6 to construct a three-dimensional tumor cell culture model in vitro. According to the type of graft, 75 male C57BL/6 mice were divided into cell control group, microcarrier control group, and experimental group (each sample corresponded to three groups, with 15 groups in total and 5 mice in each group). The liver cancer cell-microcarrier complex was implanted into the mice by subcutaneous inoculation, and tumor formation time, tumor formation rate, and histopathological manifestations were observed. The Fisher's exact test was used for comparison of categorical data between two groups. Results As for the liver cancer cells from the five patients, tumor formation was observed in the mice corresponding to three patients. In these three experiments, tumor formation was not observed in the control groups and was only observed in the experimental groups, and 12 of the 15 mice in the experimental groups had successful tumor formation, with a tumor formation rate as high as 80%, which was significantly different from that in the cell control groups and the microcarrier control groups (all P < 0.05). The tumor formation time was 5-7 days; the xenograft tumor grew rapidly, and HE staining showed nested or flaky cells with obvious heteromorphism, with the presence of pathological mitosis; immunohistochemical staining showed positive CK8/18, Hep, and Gpc-3, which was in accordance with the characteristics of human liver cancer cells. Conclusion This experiment successfully establishes a new PDX model of human liver cancer based on the complex of microcarrier 6 and human primary liver cancer cells in mice with normal immunity. This model can be used to better elucidate the mechanism of the development and progression of liver cancer in the body with normal immunity, and besides, it also provides a new animal model with higher value for the precise treatment of liver cancer. -
原发性肝癌是最常见的恶性肿瘤之一,被列为全球人类肿瘤相关死亡的第二大原因,严重威胁人类健康[1]。肝癌早期诊断仍较困难,确诊时多为晚期,术后5年生存率仅约14%[2]。尽管某些治疗形式取得了一定的成功,但肝癌的发病率和死亡率仍在迅速增加[3]。因此建立能够模拟临床特征的肝癌动物模型对于肝癌的诊治研究具有重要意义。人源肿瘤组织异种移植(patient-derived xenograft,PDX)模型是指将患者的新鲜肿瘤组织或肿瘤细胞通过原位或异位等方式移植到小鼠体内,依靠小鼠体内环境生长的一种异种移植模型[4-6]。PDX模型目前被认为是最接近临床患者的肿瘤动物模型,基于临床新鲜肝癌肿瘤标本建立的PDX模型,可以准确地反映肝癌患者的肿瘤特性。但PDX模型的构建通常选用高度免疫缺陷的小鼠,以排除机体免疫反应,但基于此模型进行肿瘤发生机制以及抗肿瘤药物的研究必然存在局限性,尤其是研究通过免疫系统来发挥作用的药物。因此,如果能在正常免疫小鼠体内成功构建人肝癌PDX模型,将对肝癌的精准医疗具有十分重要的意义。
microcarrier 6[7-9]是一种新型微载体,由可正电化有机复合多聚物组成,呈多层孔状条索样,相互交联卷曲形成有足够空间的“迷宫”样不规则结构。孔径大小、表面正电荷密度、载体颗粒大小可通过化学合成调节,是纯有机化合物,不易污染,不含杂质,具有低免疫原性、生物兼融性及可代谢性等特点,可为细胞生长提供稳定的三维微环境。microcarrier 6内部足够的空间解决了细胞生长营养和代谢废物浓度不均的问题,同时,由于microcarrier 6“迷宫”样不规则结构可以短时间内起到屏障作用,在一定程度上阻挡免疫细胞对肿瘤细胞的直接杀伤。另外,使用基质细胞衍生因子-1α(stromal cell-derived factor-1α, SDF-1α)和血管内皮生长因子(vascular endothelial growth factor,VEGF)对微载体进行修饰后,可加速血管形成,诱导血管长入,为肿瘤的快速生长提供血供营养[7]。
本研究从新鲜肝癌组织中分离提取出原代肝癌细胞,与mocrocarrier 6共培养后,将肝癌细胞-微载体复合体植入小鼠体内,在正常免疫小鼠体内成功构建了新型人肝癌PDX模型。
1. 材料和方法
1.1 主要制剂
胶原酶B购于美国Sigma公司,RPMI 1640培养基、胰酶、胎牛血清、红细胞裂解液、青链霉素均购于美国Gibco公司,兔抗人CK8/18、GPC-3、Hep单克隆抗体均购于英国Abcam公司,异氟烷购于深圳市瑞沃德生命科技有限公司。microcarrier 6由美国ELYON BIOTECHNOLOGIES LLC公司提供。
1.2 实验动物
C57BL/6小鼠75只,雄性,6~8周龄,体质量22~25 g,购于济南朋悦实验动物繁育有限公司(实验动物生产许可证编号:SCXK 20140007、实验动物使用许可证编号:SCXK 20180002),饲养于济宁医学院附属医院SPF级实验动物中心。
1.3 标本信息
5例新鲜肝癌组织标本均来自济宁医学院附属医院肝胆外科,肿瘤标本的获取经过了患者本人及家属同意,并签署知情同意书,仅供实验研究。患者详细信息见表 1。
表 1 肝癌相关PDX模型的病例信息编号 类型 性别 年龄(岁) 肿瘤大小(cm) AFP(μg/L) 分化程度 肝炎类型 有无肝硬化 LC-1 肝细胞癌 女 74 4.5×3.5×3 14.81 中 HBV 无 LC-2 胆管细胞癌 男 54 3×3×2.5 19.20 低 HBV 有 LC-3 肝细胞癌 男 44 4.5×4×3.5 56.07 中-低 HBV 有 LC-4 肝细胞癌 男 46 13.5×12×8 58 233.14 中 HBV 有 LC-5 肝细胞癌 男 60 13×12×9 6424.04 中 HBV 有 1.4 人原代肝癌细胞的获取
以RPMI 1640为溶剂配制浓度为0.05%的胶原酶B溶液,用孔径0.22 μm的滤器滤过除菌,置入37 ℃恒温水浴锅水浴30 min。将新鲜肝癌组织标本放入RPMI 1640培养基中冲洗3次,用剪刀将标本剪为体积约为1 mm×1 mm×1 mm的碎组织块,加入0.05%胶原酶B溶液,置入37 ℃恒温箱内消化1 h后取出,加入RPMI 1640稀释后反复吹打混匀,提取上清液,用孔径70 μm的滤网过滤后,以1000 r/min离心8 min。裂解红细胞后即可得人原代肝癌细胞。残余组织块中加入0.05%胶原酶B继续消化,按上述方法依次提取胶原酶B消化2、3、4 h的人原代肝癌细胞。
1.5 人原代肝癌细胞与microcarrier 6共培养
将microcarrier 6浸泡于75%酒精24 h,1×PBS缓冲液清洗3遍,加入含10%胎牛血清的RPMI 1640培养基,调整微载体悬液浓度为300 μg/mL,于37 ℃恒温箱中孵育24 h备用;用SDF-1α和VEGF对微载体进行修饰,浓度均为100 ng/mL,孵育时间为12 h。收集所有时间点提取的人原代肝癌细胞,取台盼蓝计数活细胞数>95%,取含10%胎牛血清的RPMI 1640培养基重悬细胞,调整细胞浓度为2×107/mL。1/2肝癌细胞悬液与修饰好的microcarrier 6悬液混合(体积比为1∶ 1)放于15 mL离心管中,另1/2肝癌细胞悬液加入等体积的含10%胎牛血清的RPMI 1640培养基放于15 mL离心管中,另取等体积的microcarrier 6悬液加入等量的含10%胎牛血清的RPMI 1640培养基放于15 mL离心管中,三管样本均置于37 ℃、5% CO2培养箱中培养24 h。
1.6 正常免疫小鼠肝癌PDX模型的建立
5例标本共用75只C57BL/6雄鼠,每例15只。实验分为3组,细胞对照组、微载体对照组和实验组,每组5只小鼠。细胞对照组小鼠单纯接种肝癌细胞悬液,细胞数为2×106个/只(按共培养前肝癌细胞悬液的计数);微载体对照组小鼠单纯接种空载体悬液,微载体量为30 μg/只;实验组小鼠接种肝癌细胞-微载体复合体悬液,每只小鼠接种2×106个细胞、30 μg的微载体(按共培养前肝癌细胞悬液的计数)。共培养24 h后,用1×PBS缓冲液清洗肝癌细胞、空载体和肝癌细胞-微载体复合体3遍,加入1×PBS缓冲液轻轻混匀,使最终体积跟清洗前体积一致,放于冰上备用。异氟烷麻醉小鼠,用套管针在小鼠右腋下皮下接种移植,按分组依次进行接种,200 μl/只。
1.7 观察指标及病理学检查
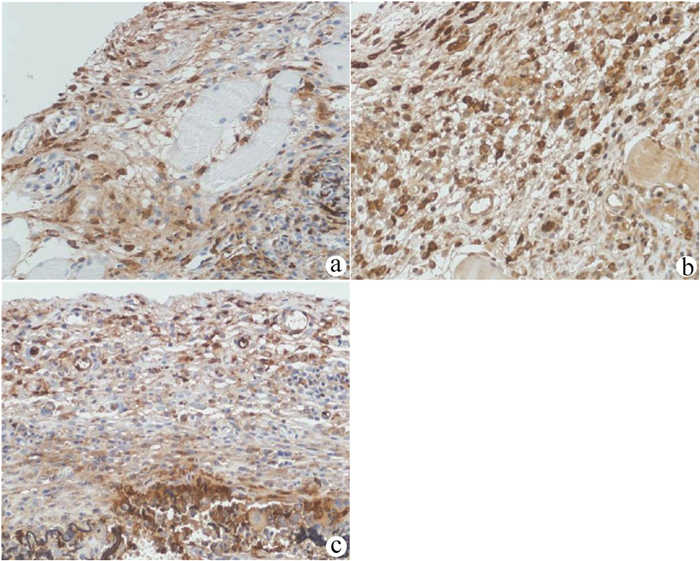
皮下接种移植的肿瘤,每周测量肿瘤最长直径(a) 和最短直径(b),计算肿瘤体积,肿瘤体积(V)= 1/2×a×b2,绘制肿瘤生长曲线。待肿瘤最长直径达1 cm,颈椎脱臼处死小鼠,完整剥离肿瘤组织,用4%中性甲醛固定,石蜡包埋切片,常规HE染色。按试剂盒说明采用EnVision二步法进行免疫组化染色。染色结果判定:阳性颗粒定位于肿瘤细胞质内,阳性细胞数<5%为阴性,阳性细胞数≥5%为阳性。
1.8 伦理学审查
本研究方案经由济宁医学院附属医院伦理委员会审批,批号:2021B029,患者均签署知情同意书;同时经由济宁医学院附属医院动物伦理委员会审批,批号:2021B029,符合实验室动物管理与使用准则。
1.9 统计学方法
使用SPSS 22.0软件进行统计学分析。计数资料两组间比较采用Fisher检验。P<0.05为差异有统计学意义。
2. 结果
2.1 基于microcarrier 6体外构建人原代肝癌细胞三维培养体系
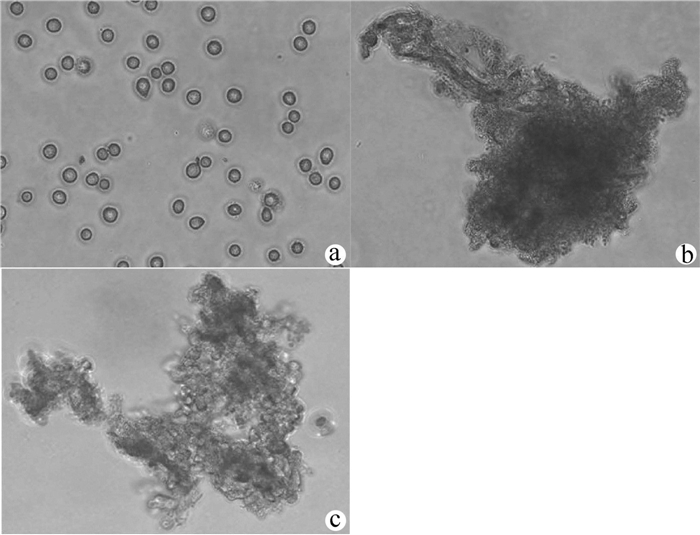
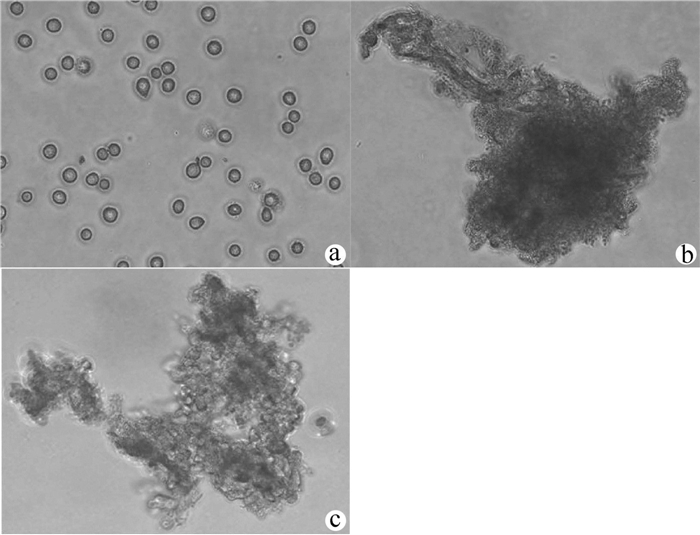
刚分离出的原代肝癌细胞呈球形,几乎全为单个细胞,折光性强(图 1a);microcarrier 6为不规则团状或长梭状,质地疏松,内部有大量孔隙(图 1b);原代肝癌细胞与修饰过的microcarrier 6共培养24 h后光镜下可看到肝癌细胞可以很好的贴附在microcarrier 6上,达到饱和状态,外周可看到不规则细胞团(图 1c)。
2.2 基于microcarrier 6在正常免疫小鼠体内建立肝癌PDX模型
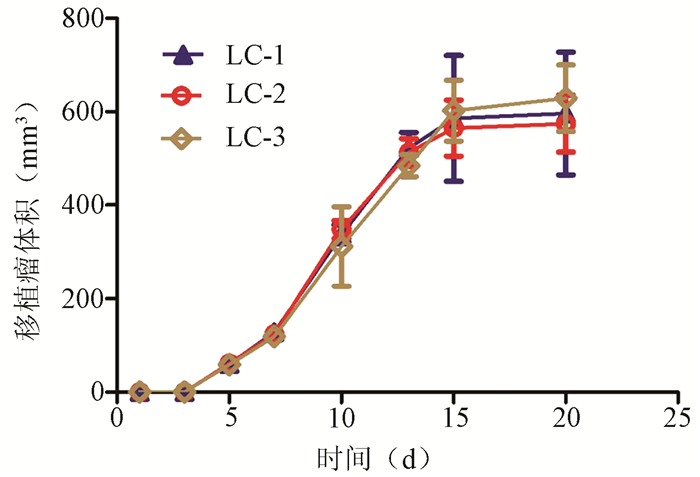
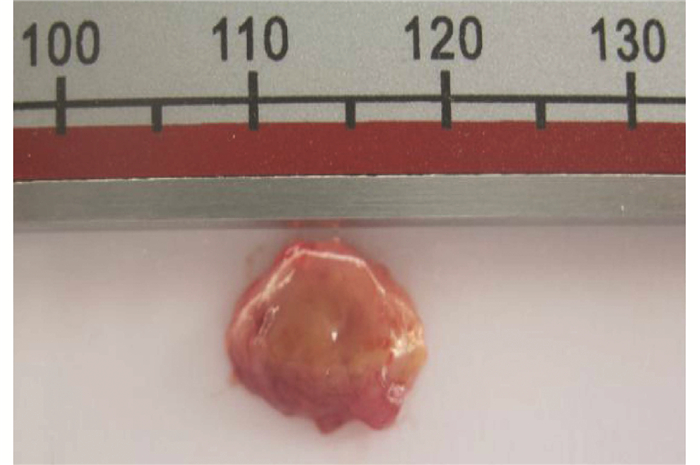
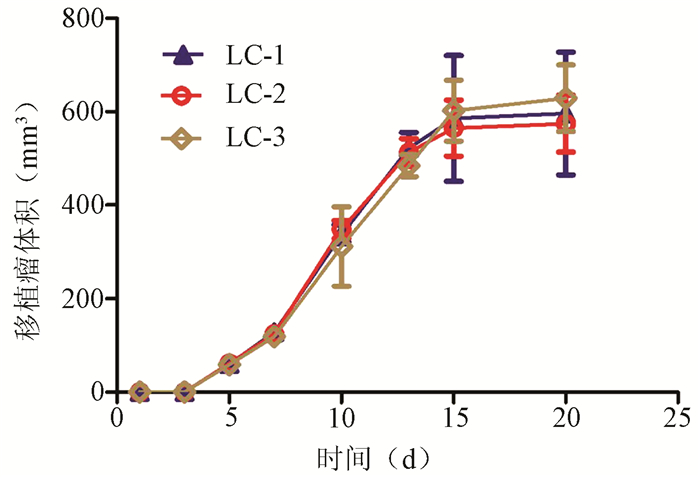
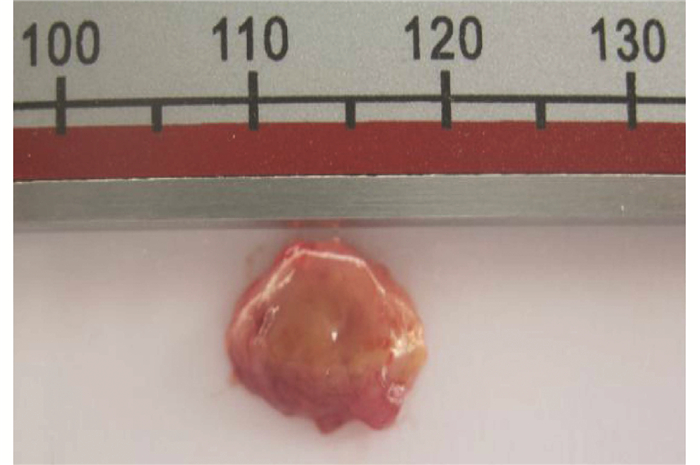
75只雄性C57BL/6小鼠均采用皮下接种法将肝癌细胞-微载体复合体植入小鼠体内,5例患者的肝癌细胞接种小鼠后有3例对应的小鼠出现了成瘤,此3例实验过程中,各组小鼠食欲、毛发、体质量无明显变化,仅实验组小鼠1周后活动度稍减少,各组没有小鼠死亡;细胞对照组和微载体对照组小鼠从实验开始到实验结束均无小鼠长出肿瘤,成瘤率均为0;实验组小鼠在接种移植后5~7 d即可触及皮下包块,实验组共有12只小鼠可触到皮下包块,总成瘤率为80%,与细胞对照组和微载体对照组比较差异均有统计学意义(P值均<0.05)(表 2)。移植瘤生长迅速,1~2周是生长高峰期,移植瘤体积迅速增大,2周左右肉眼即可观察到皮下有隆起包块,2~3周时移植瘤体积变化不大,达到稳定期,20 d左右体积可达到0.5~1 cm3(图 2、3)。移植瘤组织易与周围组织分离,形态不规整,多为圆形或椭圆形,周围血供丰富,颜色灰白色或灰红色为主(图 3)。
表 2 3例成功成瘤实验中小鼠成瘤时间和成瘤率组别 LC-1 LC-2 LC-3 成瘤时间(d) 成瘤率(%) 细胞对照组 0/5 0/5 0/5 - 01) 微载体对照组 0/5 0/5 0/5 - 01) 实验组 4/5 5/5 3/5 5~7 80 注:与实验组比较,1)P<0.05。 2.3 移植瘤组织病理学结果
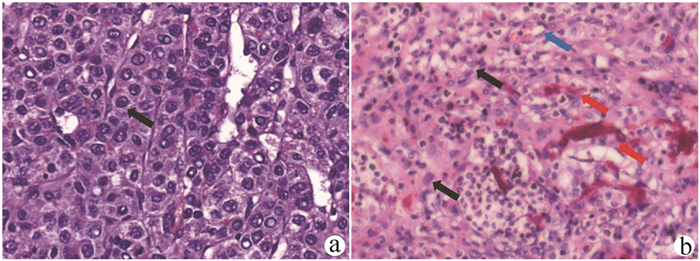
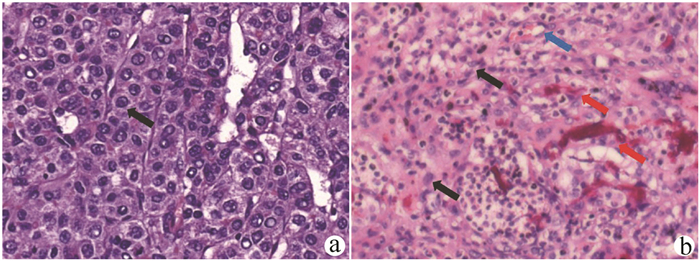
原代肝癌组织石蜡切片在光镜下可见核大深染、异形性明显、杂乱排列的肿瘤细胞(图 4a);移植瘤组织在光镜下可见大量杂乱排列、异形性明显的细胞,细胞呈圆形或椭圆形,排列呈巢状或片状,核大且大小不一,染色质粗,核仁明显,可见病理性核分裂象。间质中可见大量淋巴细胞以及尚未被清除的microcarrier 6周围的异物反应。肝癌细胞向周围浸润生长,伴有明显坏死,主要位于肿瘤中央,移植瘤组织中新生毛细血管丰富,多位于肿瘤边缘(图 4b)。
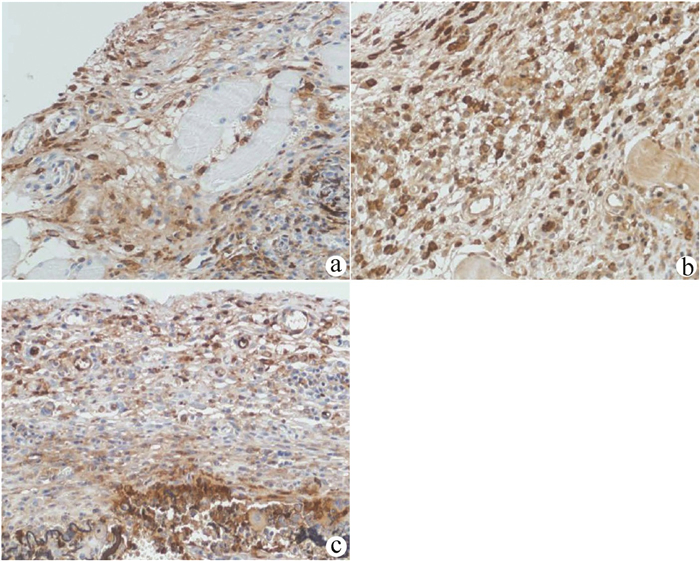
CK8/18、Hep、Gpc-3主要表达于癌细胞膜和胞质中,是肝癌细胞特异的标志物。对移植瘤组织进行免疫组化染色发现CK8/18、Hep、Gpc-3均为阳性表达(图 5),进一步证实异形性细胞为人源肝癌细胞。
3. 讨论
原发性肝癌是严重威胁人类健康的常见恶性肿瘤之一,目前肝癌的治疗方式如肝切除术或肝移植术等仅适用于早期患者,随着分子靶向治疗、免疫治疗的进展, 中晚期肝癌患者也拥有了越来越多的治疗选择[10-13],但目前仍缺乏能完全治愈肝癌的有效方法。研发针对肝癌的新型有效治疗手段均需要动物实验来进行模拟,所以建立一个模拟人肝癌的动物模型显得尤为重要。近年来随着科学技术的发展,肝癌模型的研究不断深入,目前已逐步建立小鼠肝原位移植性动物肝癌模型、诱发性肝癌模型、转基因动物及人类肝癌异种移植模型的肝癌模型[14-15]。
现有的动物模型在研究人类癌症的发生和发展上是非常宝贵的资源和工具,但目前尚缺乏理想的小鼠肝癌模型,理想的肝癌模型应能准确反映肝癌的生物特性,充分模拟人类肿瘤微环境,并且容易操作,重复性高且价廉。肝癌PDX模型是目前为止最接近临床研究的相关肿瘤模型[16-17],这种模拟人肿瘤特异性的模型对肿瘤临床前评估、治疗和预后具有重要的转化意义,有望为肿瘤患者个体化治疗带来新突破。构建PDX模型通常需要用免疫缺陷的小鼠,这些小鼠缺乏免疫系统才能避免对移植瘤的排异,但也正因为如此,这些PDX模型不能用来评价免疫相关的药物,如疫苗及免疫调节药物PD-1抗体或通过免疫激活实现的药物如CD40单抗等。此外,免疫缺陷小鼠还具有饲养费用昂贵和寿命短的缺点。
本研究选取具有正常免疫功能的C57BL/6小鼠,可以很好的解决以上问题。另外本实验选取的4例临床样本病理类型均为肝细胞癌,是最常见的原发性肝癌类型,具有临床代表性。移植部位和途径选择小鼠腋下皮下异位移植,主要考虑该部位血供丰富和组织疏松有利于肿瘤的生长[18]。
本研究采用microcarrier 6是一类新型微载体,人原代肝癌细胞与之共培养可以成功构建三维生长模型。microcarrier 6具有低免疫源性、质地疏松的特点,中间有大量孔隙可供肿瘤细胞在其中生长,可以(短时间内)起到屏障作用,阻挡免疫细胞直接杀伤肿瘤细胞,并且经过修饰后有利于血管生成,为肿瘤细胞快速生长提供了良好的条件[7-9]。肿瘤细胞与microcarrier 6共同培养24 h后形成的3D细胞团,既能避免单个肿瘤细胞进入小鼠体内后被小鼠的免疫系统迅速清除,也能克服移植原代组织块后细胞由于缺乏血供而死亡的缺陷。本课题组前期不仅使用MKN45细胞系成功构建胃癌小鼠移植瘤模型,并利用人原代卵巢细胞构建了正常免疫小鼠的人原代卵巢癌细胞模型[7-9]。
借助于本研究构建的人原代肝癌细胞的三维生长模型,实验组在正常免疫小鼠体内成功构建了人肝癌PDX模型,而细胞对照组和微载体对照组均未成瘤。该模型特点是肿瘤生长迅速,在5~7 d时可以触摸到瘤块,1~2周为肿瘤生长高峰期,20 d左右体积即可达到0.5~1.0 cm3。病理HE染色可见大量核异型性细胞,并向肌肉、脂肪等浸润;残存的microcarrier 6被淋巴细胞包绕形成肉芽肿;中央有大片坏死,考虑生长过快,血供不足所致,符合人体肝癌的发展规律;毛细血管丰富,主要位于肿瘤周边。肝癌特异性标志物CK8/18、Gpc-3、Hep的免疫组化染色均为阳性,进一步证实异形性细胞为人肝癌细胞。
本实验成功基于microcarrier 6复合人原代肝癌细胞在正常免疫小鼠体内建立了人肝癌PDX模型,该模型可用于体现机体免疫系统和肿瘤的相互作用,在未来实现肿瘤转化研究及精准治疗等方面势必有着广泛的前景。
-
表 1 肝癌相关PDX模型的病例信息
编号 类型 性别 年龄(岁) 肿瘤大小(cm) AFP(μg/L) 分化程度 肝炎类型 有无肝硬化 LC-1 肝细胞癌 女 74 4.5×3.5×3 14.81 中 HBV 无 LC-2 胆管细胞癌 男 54 3×3×2.5 19.20 低 HBV 有 LC-3 肝细胞癌 男 44 4.5×4×3.5 56.07 中-低 HBV 有 LC-4 肝细胞癌 男 46 13.5×12×8 58 233.14 中 HBV 有 LC-5 肝细胞癌 男 60 13×12×9 6424.04 中 HBV 有 表 2 3例成功成瘤实验中小鼠成瘤时间和成瘤率
组别 LC-1 LC-2 LC-3 成瘤时间(d) 成瘤率(%) 细胞对照组 0/5 0/5 0/5 - 01) 微载体对照组 0/5 0/5 0/5 - 01) 实验组 4/5 5/5 3/5 5~7 80 注:与实验组比较,1)P<0.05。 -
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492. [2] LI L, CHEN J, CHEN X, et al. Serum miRNAs as predictive and preventive biomarker for pre-clinical hepatocellular carcinoma[J]. Cancer Lett, 2016, 373(2): 234-240. DOI: 10.1016/j.canlet.2016.01.028. [3] SIEGEL RL, MILLER KD, JEMAL A. Cancer Statistics, 2017[J]. CA Cancer J Clin, 2017, 67(1): 7-30. DOI: 10.3322/caac.21387. [4] IZUMCHENKO E, MEIR J, BEDI A, et al. Patient-derived xenografts as tools in pharmaceutical development[J]. Clin Pharmacol Ther, 2016, 99(6): 612-621. DOI: 10.1002/cpt.354. [5] POMPILI L, PORRU M, CARUSO C, et al. Patient-derived xenografts: A relevant preclinical model for drug development[J]. J Exp Clin Cancer Res, 2016, 35(1): 189. DOI: 10.1186/s13046-016-0462-4. [6] XU C, LI X, LIU P, et al. Patient-derived xenograft mouse models: A high fidelity tool for individualized medicine[J]. Oncol Lett, 2019, 17(1): 3-10. DOI: 10.3892/ol.2018.9583. [7] BI YZ, KONG LB, GAO PF, et al. Construction of a normal immune mouse human gastric cancer transplantation model based on microcarrier 6[J]. Chin J Clin Oncol, 2017, 44(5): 199-203. DOI: 10.3969/j.issn.1000-8179.2017.05.168.毕研贞, 孔令斌, 高鹏飞, 等. 基于microcarrier 6构建正常免疫小鼠人胃癌移植模型[J]. 中国肿瘤临床, 2017, 44(5): 199-203. DOI: 10.3969/j.issn.1000-8179.2017.05.168. [8] WANG QY, SUN FQ, BI YZ, et al. Establishment of microcarrier human gastric cancer animal model and discussion of pathological characteristics[J]. Chin J Gen Surg, 2017, 32(10): 895-896. DOI: 10.3760/cma.j.issn.1007-631X.2017.10.031.王全义, 孙富强, 毕研贞, 等. 微载体人胃癌动物模型的建立及其病理特征的探讨[J]. 中华普通外科杂志, 2017, 32(10): 895-896. DOI: 10.3760/cma.j.issn.1007-631X.2017.10.031. [9] HUANG R, HONG F, BI YZ, et al. Establishment of primary ovarian cancer model in mice with normal immunity[J]. Progr Obstetr Gynecol, 2018, 27(12): 901-904. DOI: 10.13283/j.cnki.xdfckjz.2018.12.004.黄蓉, 洪丰, 毕研贞, 等. 正常免疫鼠人原代卵巢癌模型的建立[J]. 现代妇产科进展, 2018, 27(12): 901-904. DOI: 10.13283/j.cnki.xdfckjz.2018.12.004. [10] KUDO M. Systemic therapy for hepatocellular carcinoma: 2017 update[J]. Oncology, 2017, 93(Suppl 1): 135-146. DOI: 10.1159/000481244. [11] GRANDHI MS, KIM AK, RONNEKLEIV-KELLY SM, et al. Hepatocellular carcinoma: From diagnosis to treatment[J]. Surg Oncol, 2016, 25(2): 74-85. DOI: 10.1016/j.suronc.2016.03.002. [12] RICH NE, YOPP AC, SINGAL AG. Medical management of hepatocellular carcinoma[J]. J Oncol Pract, 2017, 13(6): 356-364. DOI: 10.1200/JOP.2017.022996. [13] ZHU J, YIN T, XU Y, et al. Therapeutics for advanced hepatocellular carcinoma: Recent advances, current dilemma, and future directions[J]. J Cell Physiol, 2019, 234(8): 12122-12132. DOI: 10.1002/jcp.28048. [14] YAN Y, CHEN N, WANG Y, et al. The application of antitumor drug-targeting models on liver cancer[J]. Drug Deliv, 2016, 23(5): 1667-1675. DOI: 10.3109/10717544.2015.1064188. [15] WEBER A, O'CONNOR T, HEIKENWALDER M. Next generation of preclinical liver cancer models[J]. Clin Cancer Res, 2015, 21(19): 4254-4256. DOI: 10.1158/1078-0432.CCR-15-1152. [16] OKADA S, VAETEEWOOTTACHARN K, KARIYA R. Establishment of a patient-derived tumor xenograft model and application for precision cancer medicine[J]. Chem Pharm Bull (Tokyo), 2018, 66(3): 225-230. DOI: 10.1248/cpb.c17-00789. [17] CHO SY, KANG W, HAN JY, et al. An integrative approach to precision cancer medicine using patient-derived xenografts[J]. Mol Cells, 2016, 39(2): 77-86. DOI: 10.14348/molcells.2016.2350. [18] ZHENG MJ, WANG J, CHEN YW, et al. A novel mouse model of gastric cancer with human gastric microenvironment[J]. Cancer Lett, 2012, 325(1): 108-115. DOI: 10.1016/j.canlet.2012.06.011. 期刊类型引用(0)
其他类型引用(2)
-




 PDF下载 ( 3030 KB)
PDF下载 ( 3030 KB)

 下载:
下载:





 下载:
下载: