Clinical features and prognosis of primary myelofibrosis with hepatic vascular disease and/or portal hypertension
-
摘要:
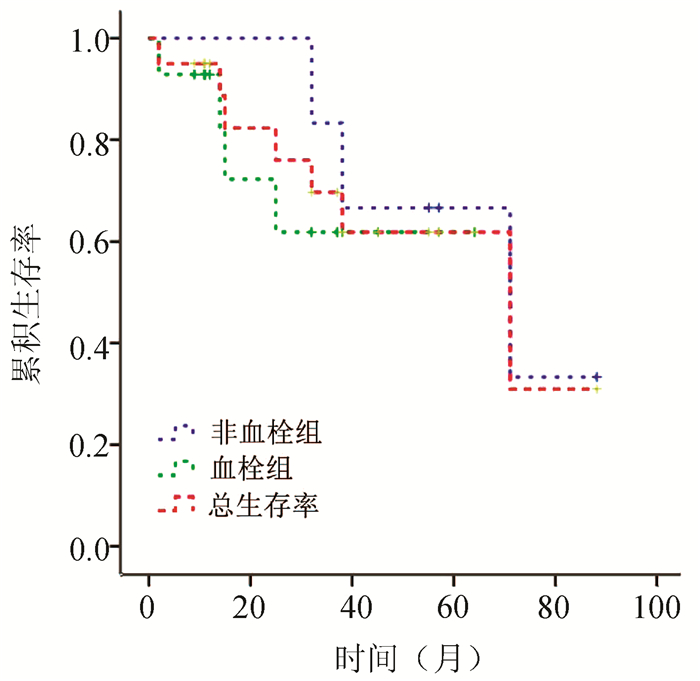
目的 探讨原发性骨髓纤维化(PMF)相关肝血管疾病和门静脉高压症的临床特征、肝组织病理学特点和预后。 方法 选取2010年7月—2020年12月在解放军第九六〇医院和空军军医大学西京消化病医院确诊的68例PMF患者,其中22例以肝血管疾病/门静脉高压症为主要表现,回顾性分析了这些患者的临床和病理特征。根据患者有无血栓形成分为两组,总结其治疗和预后情况。通过Kaplan-Meier绘制生存曲线,通过log-rank检验比较两组间长期生存率。 结果 68例PMF患者中,22例出现肝血管疾病和/或门静脉高压症,患病率约为32.35%。其中,13例(59.1%)表现为门静脉血栓形成,1例(4.5%)表现为Budd-Chiari综合征,8例(36.4%)表现为门静脉高压症。7例患者接受肝活检,病理均显示肝脏髓外造血,小叶和汇管区淋巴细胞、浆细胞和嗜酸性粒细胞不同程度浸润,但肝小叶结构正常。随访期间7例死亡患者,其中5例死于血栓形成或门静脉高压相关并发症。患者总体中位生存时间为57.99个月,其中血栓形成组中位生存时间为45.33个月,无血栓组中位生存时间为64.00个月,尽管没有显著统计学差异(χ2=3.035,P=0.081),但是可以看到非血栓组较血栓组表现出更好的生存趋势。 结论 肝血管疾病和门静脉高压症患者应考虑原发病为PMF的可能性,而PMF患者也应进行肝血管疾病筛查,并早期干预。无血栓形成的患者可能比血栓形成的患者有更好的生存趋势。 Abstract:Objective To investigate the clinical features, liver histopathological features, treatment, and prognosis of primary myelofibrosis (PMF)-associated hepatic vascular disease and portal hypertension. Methods A retrospective analysis was performed for the clinical and pathological features of 68 patients who were diagnosed with PMF in 960 Hospital of the PLA Joint Logistics Support Force and Xijing Hospital of Digestive Diseases, Air Force Medical University, from July 2010 to December 2020, among whom 22 patients had hepatic vascular disease/portal hypertension as the main manifestation. The patients were divided into two groups according to the presence or absence of thrombosis, and treatment and prognosis were summarized. The Kaplan-Meier method was used to plot survival curves, and the log-rank test was used to compare long-term survival rate between the two groups. Results Among the 68 patients with PMF, 22 had hepatic vascular disease and/or portal hypertension, resulting in a prevalence rate of 32.35%, and among these 22 patients, 13 (59.1%) had extrahepatic portal vein occlusion, 1 (4.5%) had Budd-Chiari syndrome, and 8 (36.4%) had portal hypertension. Biopsy was performed for 7 patients, and pathological results showed extramedullary hematopoiesis in the liver and varying degrees of infiltration of lymphocytes, plasma cells, and eosinophils at the lobular and portal areas, but the lobular structure was normal. A total of 7 patients died during follow-up, among whom 5 died of complications associated with thrombosis or portal hypertension. The overall median survival time was 57.99 months for all patients; the median survival time was 45.33 months in patients with thrombosis and 64.00 months in patients without thrombosis, and although there was no significant difference between the two groups (χ2=3.035, P=0.081), the non-thrombosis group tended to have better survival and prognosis than the thrombosis group. Conclusion The possibility of PMF as the primary disease should be considered for patients with hepatic vascular disease and portal hypertension. Patients with PMF should be screened for hepatic vascular disease, and early intervention should be given. The patients without thrombosis tend to have better survival and prognosis than those with thrombosis. -
Key words:
- Primary Myelofibrosis /
- Hypertension, Portal /
- Venous Thrombosis /
- Therapeutics /
- Prognosis
-
表 1 22例PMF并发肝血管疾病/门静脉高压症患者的临床特征
变量 数值 年龄(岁) 54(23~77) 性别[例(%)] 男性 9 (40.91) 女性 13(59.09) 门静脉高压症的病因(例) Budd-Chiari综合征 1 PVT 13 单纯门静脉高压症 8 初诊症状(例) 食管胃底静脉曲张出血 3 腹水 7 食管胃底静脉曲张出血+腹水 3 腹痛 5 黄疸 1 乏力 3 脾肿大(例) 22 肝肿大(例) 3 血常规 血红蛋白(g/dL) 103 (56~156) 白细胞(×109/L) 11.14 (1.37~38.15) 血小板(×109/L) 201 (17~1028) 肝功能 AST (U/L) 23 (6~53) ALT (U/L) 14 (6~69) 总胆红素(mg/dL) 18.6 (8.8~85.0) 凝血时间(s) 15 (13~18) 白蛋白(g/dL) 39.6 (28.4~46.1) 基因突变检测[例(%)] JAK2 8/14 (57.14) CALR 3/11 (27.27) MTHFR(SNP) 6/7 (85.71) 表 2 PMF并发肝血管疾病/门静脉高压症患者治疗情况
治疗情况 例数 血栓组(n=14) 非血栓组(n=8) 抗凝和溶栓 11 11 0 NSBB 2 0 2 TIPS 5 2 3 Ruxolitinib 1 0 1 未治疗 3 1 2 -
[1] ABU-HILAL M, TAWAKER J. Portal hypertension secondary to myelofibrosis with myeloid metaplasia: A study of 13 cases[J]. World J Gastroenterol, 2009, 15(25): 3128-3133. DOI: 10.3748/wjg.15.3128. [2] POTTHOFF A, ATTIA D, PISCHKE S, et al. Long-term outcome of liver transplant patients with Budd-Chiari syndrome secondary to myeloproliferative neoplasms[J]. Liver Int, 2015, 35(8): 2042-2049. DOI: 10.1111/liv.12816. [3] SOKOLOVA M, TSVETAEVA N, SUKHANOVA G, et al. Ph-negative chronic myeloproliferative neoplasm (primary myelofibrosis) - as one of the reasons of the budd-Chiari syndrome[J]. Mediterr J Hematol Infect Dis, 2012, 4(1): e2012047. DOI: 10.4084/MJHID.2012.047. [4] CERVANTES F, BAROSI G, HERNÁNDEZ-BOLUDA JC, et al. Myelofibrosis with myeloid metaplasia in adult individuals 30 years old or younger: Presenting features, evolution and survival[J]. Eur J Haematol, 2001, 66(5): 324-327. DOI: 10.1034/j.1600-0609.2001.066005324.x. [5] QI X, JIA J, BAI M, et al. Portal hypertension complicating myelofibrosis in a patient without portal or hepatic vein thrombosis[J]. Ann Gastroenterol, 2014, 27(2): 188. [6] VAHABZADEH B, CRIPPIN JS. Image of the month. Portal hypertension as a result of extramedullary hematopoiesis caused by idiopathic myelofibrosis[J]. Clin Gastroenterol Hepatol, 2008, 6(4): e22. DOI: 10.1016/j.cgh.2008.01.008. [7] REILLY CR, BABUSHOK DV, MARTIN K, et al. Multicenter analysis of the use of transjugular intrahepatic portosystemic shunt for management of MPN-associated portal hypertension[J]. Am J Hematol, 2017, 92(9): 909-914. DOI: 10.1002/ajh.24798. [8] DOKI N, IRISAWA H, TAKADA S, et al. Transjugular intrahepatic portosystemic shunt for the treatment of portal hypertension due to idiopathic myelofibrosis[J]. Intern Med, 2007, 46(4): 187-190. DOI: 10.2169/internalmedicine.46.1768. [9] ALVAREZ-LARRÁN A, ABRALDES JG, CERVANTES F, et al. Portal hypertension secondary to myelofibrosis: A study of three cases[J]. Am J Gastroenterol, 2005, 100(10): 2355-2358. DOI: 10.1111/j.1572-0241.2005.50374.x. [10] WIEST R, STRAUCH U, WAGNER H, et al. A patient with myelofibrosis complicated by refractory ascites and portal hypertension: To tips or not to tips? A case report with discussion of the mechanism of ascites formation[J]. Scand J Gastroenterol, 2004, 39(4): 389-394. DOI: 10.1080/00365520310007521. [11] BĚLOHLÁVEK J, SCHWARZ J, JIRÁSEK A, et al. Idiopathic myelofibrosis complicated by portal hypertension treated with a transjugular intrahepatic portosystemic shunt (TIPS)[J]. Wien Klin Wochenschr, 2001, 113(5-6): 208-211. [12] HOW J, ZHOU A, OH ST. Splanchnic vein thrombosis in myeloproliferative neoplasms: Pathophysiology and molecular mechanisms of disease[J]. Ther Adv Hematol, 2017, 8(3): 107-118. DOI: 10.1177/2040620716680333. [13] TAN HK, LEOW WQ, CHANG PE. Ruxolitinib for the treatment of portal hypertension in a patient with primary myelofibrosis[J]. Gastroenterology, 2019, 157(5): e26-e27. DOI: 10.1053/j.gastro.2016.08.059. [14] PANDA A, CHANDRASHEKHARA SH, NAMBIRAJAN A, et al. Idiopathic myelofibrosis with disseminated hepatosplenic, mesenteric, renal and pulmonary extramedullary haematopoeisis, portal hypertension and tuberculosis: Initial presentation and 2 years follow-up[J]. BMJ Case Rep, 2016, 2016. DOI: 10.1136/bcr-2016-217854. [15] DURCHSCHEIN F, KRONES E, EHERER AJ, et al. Sclerotherapy-associated esophageal hematoma in a patient with myelofibrosis and portal hypertension[J]. Endoscopy, 2015, 47(Suppl 1)UCTN: e20-e21. DOI: 10.1055/s-0034-1390725. [16] TOKAI K, MIYATANI H, YOSHIDA Y, et al. Multiple esophageal variceal ruptures with massive ascites due to myelofibrosis-induced portal hypertension[J]. World J Gastroenterol, 2012, 18(28): 3770-3774. DOI: 10.3748/wjg.v18.i28.3770. [17] de FRANCHIS R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension[J]. J Hepatol, 2015, 63(3): 743-752. DOI: 10.1016/j.jhep.2015.05.022. [18] BARBUI T, THIELE J, GISSLINGER H, et al. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: Document summary and in-depth discussion[J]. Blood Cancer J, 2018, 8(2);15. DOI: 10.1038/s41408-018-0054-y. [19] RUMI E, CAZZOLA M. Diagnosis, risk stratification, and response evaluation in classical myeloproliferative neoplasms[J]. Blood, 2017, 129(6): 680-692. DOI: 10.1182/blood-2016-10-695957. [20] TEFFERI A. Primary myelofibrosis: 2019 update on diagnosis, risk-stratification and management[J]. Am J Hematol, 2018, 93(12): 1551-1560. DOI: 10.1002/ajh.25230. [21] SILVERSTEIN MN, WOLLAEGER EE, BAGGENSTOSS AH. Gastrointestinal and abdominal manifestations of agnogenic myeloid metaplasia[J]. Arch Intern Med, 1973, 131(4): 532-537. DOI: 10.1001/archinte.1973.00320100060007 [22] LIGUMSKI M, POLLIACK A, BENBASSAT J. Nature and incidence of liver involvement in agnogenic myeloid metaplasia[J]. Scand J Haematol, 1978, 21(2): 81-93. DOI: 10.1111/j.1600-0609.1978.tb02497.x. [23] VANNUCCHI AM. Insights into the pathogenesis and management of thrombosis in polycythemia vera and essential thrombocythemia[J]. Intern Emerg Med, 2010, 5(3): 177-184. DOI: 10.1007/s11739-009-0319-3. [24] FUJIYAMA S, SAITOH S, KAWAMURA Y, et al. Portal vein thrombosis in liver cirrhosis: Incidence, management, and outcome[J]. BMC Gastroenterol, 2017, 17(1): 112. DOI: 10.1186/s12876-017-0668-8. [25] QI X, HAN G, FAN D. Management of portal vein thrombosis in liver cirrhosis[J]. Nat Rev Gastroenterol Hepatol, 2014, 11(7): 435-446. DOI: 10.1038/nrgastro.2014.36. [26] DELEVE LD, VALLA DC, GARCIA-TSAO G. Vascular disorders of the liver[J]. Hepatology, 2009, 49(5): 1729-1764. DOI: 10.1002/hep.22772. [27] SONG ZQ, ZHOU LY. A clinical analysis of six cases of portal hypertension secondary to primary myelofibrosis and review of literatures[J]. Chin J Intern Med, 2010, 49(10): 845-847. DOI: 10.3760/cma.j.issn.0578-1426.2010.10.009.宋志强, 周丽雅. 原发性骨髓纤维化继发门脉高压症六例分析并文献复习[J]. 中华内科杂志, 2010, 49(10): 845-847. DOI: 10.3760/cma.j.issn.0578-1426.2010.10.009. [28] WANG X, HE TT, ZHANG N, et al. Clinical characteristics of 12 patients with primary myelofibrosis and liver cirrhosis[J]. J Clin Hepatol, 2020, 36(11): 2524-2526. DOI: 10.3969/j.issn.1001-5256.2020.11.025.王宣, 何婷婷, 张宁, 等. 12例原发性骨髓纤维化合并肝硬化患者的临床特征分析[J]. 临床肝胆病杂志, 2020, 36(11): 2524-2526. DOI: 10.3969/j.issn.1001-5256.2020.11.025. -



 PDF下载 ( 2515 KB)
PDF下载 ( 2515 KB)

 下载:
下载: