血清游离三甲碘状腺原氨酸水平预测HBV相关慢加急性肝衰竭患者预后的价值分析
DOI: 10.3969/j.issn.1001-5256.2022.01.012
Value of serum free triiodothyronine level in predicting the prognosis of patients with HBV-related acute-on-chronic liver failure
-
摘要:
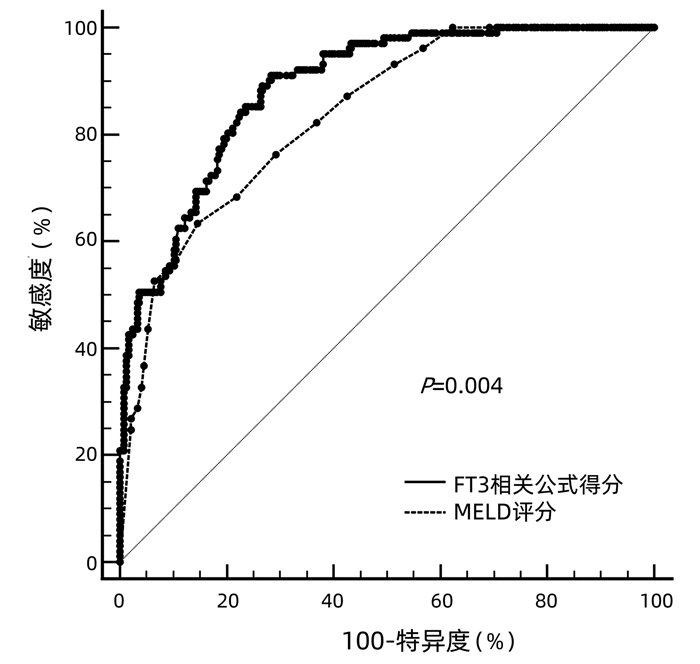
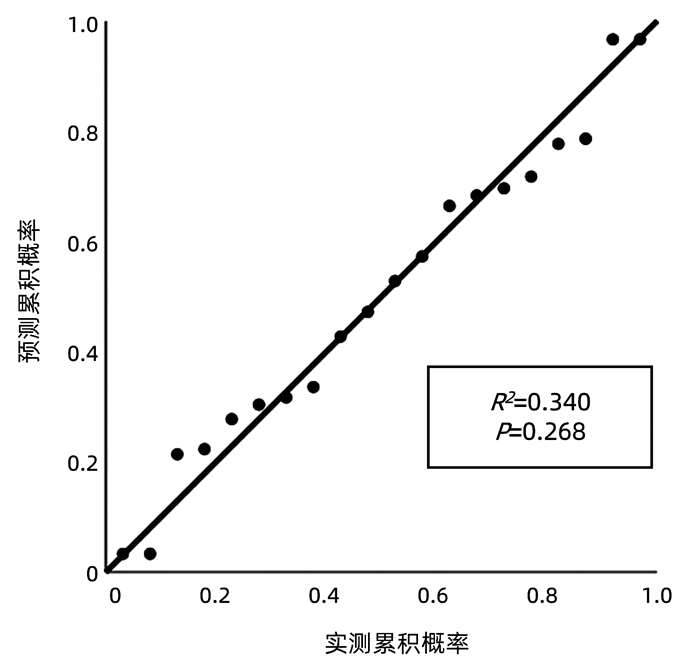
目的 探讨血清游离三甲碘状腺原氨酸(FT3)水平对HBV相关慢加急性肝衰竭(HBV-ACLF)患者90 d预后的预测价值。 方法 收集2018年9月—2020年1月在首都医科大学附属北京佑安医院住院的122例HBV-ACLF患者的临床资料,根据确诊后90 d预后分为生存组(n=77)和死亡组(n=45)。采用ELISA法测定血清FT3水平,比较FT3水平在两组间差异,应用logistic回归分析探索影响预后的危险因素并建立FT3相关预测模型,采用预测概率值的ROC曲线下面积(AUC)评估预测模型的区分度,采用线性回归分析评估校准度。采用AUC比较模型与MELD评分预测预后价值的差异。符合正态分布的计量资料两组间比较采用t检验;非正态分布的计量资料两组间比较采用Mann-Whitney U检验;计数资料两组间比较采用χ2检验;采用logistic回归分析预后影响因素。 结果 死亡组FT3显著低于生存组[(2.27±0.38) pmol/L vs (2.69±0.55) pmol/L,t=4.526, P<0.001], FT3(OR=0.534,95%CI:0.300~0.950,P=0.013)是影响预后的独立保护因素;年龄(OR=1.047,95%CI:1.013~1.082,P=0.007)、TBil(OR=1.096,95%CI:1.059~1.134,P<0.001)、INR(OR=1.101,95%CI:1.029~1.178,P<0.005)、Cr(OR=4.583,95%CI:2.102~7.992,P<0.001)是独立危险因素。FT3相关预测模型区分度的AUC为0.869(95%CI:0.831~0.907),P<0.001;校准能力R2=0.340,P=0.268。FT3相关公式预测预后的价值显著好于MELD评分(P<0.05)。 结论 FT3是HBV-ACLF患者90 d预后的独立影响因素,其与年龄、TBil、INR、Cr联合建立的FT3相关预测模型对患者90 d预后评估具有较好的预测价值。 Abstract:Objective To investigate the value of serum free triiodothyronine (FT3) level in predicting the 90-day prognosis of patients with hepatitis B virus (HBV)-related acute-on-chronic liver failure (ACLF). Methods Related clinical data were collected from 122 patients with HBV-ACLF who were hospitalized in Beijing YouAn Hospital, Capital Medical University, from September 2018 to January 2020, and according to their prognosis on day 90 after confirmed diagnosis, they were divided into survival group with 77 patients and death group with 45 patients. ELISA was used to measure the serum level of FT3, which was then compared between the two groups; a logistic regression analysis was used to investigate the risk factors for prognosis and establish an FT3-related predictive model; the area under the receiver operating characteristic (ROC) curve (AUC) of the predicted probability value was used to evaluate the discriminatory ability of the predictive model, and a linear regression analysis was used to evaluate calibration degree. AUC was used to compare the predictive value of this model and Model for End-Stage Liver Disease (MELD) score. The t-test was used for comparison of normally distributed continuous data between two groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups; the chi-square test was used for comparison of categorical data between two groups; univariate and multivariate logistic regression analyses were used to investigate the influencing factors for prognosis. Results The death group had a significantly lower serum level of FT3 than the survival group (2.27±0.38 pmol/L vs 2.69±0.55 pmol/L, t=4.526, P < 0.001). FT3 (odds ratio [OR]=0.534, 95% confidence interval [CI]: 0.300-0.950, P=0.013) was an independent protective factor against poor prognosis, while age (OR=1.047, 95%CI: 1.013-1.082, P=0.007), total bilirubin (TBil) (OR=1.096, 95%CI: 1.059-1.134, P < 0.001), international normalized ratio (INR) (OR=1.101, 95%CI: 1.029-1.178, P < 0.005), and creatinine (Cr) (OR=4.583, 95%CI: 2.102-7.992, P < 0.001) were independent risk factors. In terms of discriminatory ability, the FT3-related predictive model had an AUC of 0.869 (95%CI: 0.831-0.907, P < 0.001), and for calibration ability, R2=0.340, P=0.268. The FT3-related formula was better than MELD score in predicting prognosis (P < 0.05). Conclusion FT3 is an independent influencing factor for 90-day prognosis in patients with HBV-ACLF, and the FT3-related predictive model based on FT3 in combination with age, TBil, INR, and Cr has a good value in predicting 90-day prognosis. -
Key words:
- Acute-On-Chronic Liver Failure /
- Triiodothyronine /
- Prognosis
-
表 1 HBV-ACLF患者入院情况
指标 生存组(n=77) 死亡组(n=45) 统计值 P值 年龄(岁) 44.65±10.58 48.85±10.13 t=4.551 <0.001 男/女(例) 65/12 35/10 χ2=1.301 0.254 慢性乙型肝炎/肝硬化(例) 31/46 14/31 χ2=7.345 0.007 ALT(U/L) 285.81(112.27~521.15) 153.65(92.17~3 756.18) U=1.179 0.230 AST(U/L) 153.50(92.95~434.76) 136.9(86.1~305.95) U=1.016 0.618 TBil(mg/dL) 19.26±8.59 28.31±8.97 t=9.375 <0.001 INR 2.73±1.27 5.23±1.86 t=4.859 <0.001 Cr(mg/dL) 0.72±0.25 1.54±0.56 t=7.914 <0.001 Alb(g/L) 32.34±3.95 31.72±4.19 t=-1.295 0.196 Na(mmol/L) 136.22±3.67 133.65±6.91 t=-4.514 <0.001 WBC(×109/L) 7.40(5.01~9.57) 9.19(6.04~10.56) U=8.573 0.001 MELD 26.48±5.19 33.68±4.87 t=11.950 <0.001 表 2 预后危险因素的单因素logistic回归分析
因素 β值 Wald值 OR值 95%CI P值 年龄 0.054 18.305 1.055 1.030~1.082 <0.001 性别 0.500 1.279 1.148 0.693~2.917 0.258 慢性乙型肝炎/肝硬化 -0.689 7.213 0.502 0.303~0.830 0.007 TBil 0.114 56.431 1.121 1.088~1.165 <0.001 INR 0.193 5.594 1.213 1.034~1.423 0.018 Alb -0.038 1.669 0.962 0.908~1.020 0.196 Cr 2.226 28.933 9.259 4.115~14.834 <0.001 Na -0.108 17.818 0.898 0.854~0.944 <0.001 FT3 -0.982 18.478 0.375 0.240~0.586 <0.001 WBC 0.070 9.919 1.073 1.027~1.120 0.002 表 3 预后危险因素的多因素logistic回归分析
因素 β值 Wald值 OR值 95%CI P值 年龄 0.046 7.330 1.047 1.013~1.082 0.007 慢性乙型肝炎/肝硬化 -0.595 2.922 0.552 0.279~1.091 0.097 TBil 0.092 27.979 1.096 1.059~1.134 <0.001 INR 0.096 7.797 1.101 1.029~1.178 0.005 Cr 1.522 14.649 4.583 2.102~7.992 <0.001 Na -0.043 1.991 0.958 0.902~1.017 0.158 FT3 -0.627 4.559 0.534 0.300~0.950 0.013 WBC 0.011 0.159 1.011 0.956~1.070 0.690 -
[1] Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association, Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35(1): 38-44. DOI: 10.3969/j.issn.1001-5256.2019.01.007.中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2018年版)[J]. 临床肝胆病杂志, 2019, 35(1): 38-44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. [2] LIU CR, LI YP, LUO S, et al. Influencing factors for the short-term prognosis of patients with HBV-related acute-on-chronic liver failure[J]. J Clin Hepatol, 2021, 37(1): 56-62. DOI: 10.3969/j.issn.1001-5256.2021.01.012.刘晨瑞, 李亚萍, 罗森, 等. HBV相关慢加急性肝衰竭患者短期预后的影响因素分析[J]. 临床肝胆病杂志, 2021, 37(1): 56-62. DOI: 10.3969/j.issn.1001-5256.2021.01.012. [3] XU J, HUANG M. Predictive value of plasma diamine oxidase concentration combined with iMELD score on short-term prognosis of hepatitis B virus-related acute-on-chronic liver failure[J/CD]. Chin J Liver Dis (Electronic Version), 2020, 12(2): 68-75. DOI: 10.3969/j.issn.1674-7380.2020.02.012.许俊, 黄敏. 血浆二胺氧化酶联合iMELD评分对乙型肝炎病毒相关慢加急性肝衰竭患者近期预后的预测价值[J/CD]. 中国肝脏病杂志(电子版), 2020, 12(2): 68-75. DOI: 10.3969/j.issn.1674-7380.2020.02.012. [4] LI ZL, TONG XJ, TIAN WW, et al. Expression and clinical significance of interleukin 26 in patients with hepatitis B virus related acute-on-chronic liver failure[J]. Clin J Med Offic, 2020, 48(12): 1492-1493, 1496. DOI: 10.16680/j.1671-3826.2020.12.39.李增莲, 仝晓净, 田稳稳, 等. 白细胞介素26在乙型肝炎病毒相关慢加急性肝衰竭患者中表达及临床意义[J]. 临床军医杂志, 2020, 48(12): 1492-1493, 1496. DOI: 10.16680/j.1671-3826.2020.12.39. [5] PIANTANIDA E, IPPOLITO S, GALLO D, et al. The interplay between thyroid and liver: Implications for clinical practice[J]. J Endocrinol Invest, 2020, 43(7): 885-899. DOI: 10.1007/s40618-020-01208-6. [6] KAN YT, YANG YF. Serum thyroid hormones in acute on chronic liver failure patients and its correlation with prognosis[J]. Chin Hepatol, 2018, 23(4): 305-306. DOI: 10.3969/j.issn.1008-1704.2018.04.009.阚延婷, 杨永峰. 慢加急性肝衰竭患者血清甲状腺激素水平变化及其与预后的关系[J]. 肝脏, 2018, 23(4): 305-306. DOI: 10.3969/j.issn.1008-1704.2018.04.009. [7] ZHAO WM, DONG PL, FAN CL, et al. The clinical value of thyroid function in patients with acute-on-chronic liver failure[J]. Beijing Med J, 2020, 42(6): 554-556. DOI: 10.15932/j.0253-9713.2020.06.018.赵文敏, 董培玲, 范春蕾, 等. 慢加急(亚急)肝衰竭患者甲状腺功能变化及临床意义[J]. 北京医学, 2020, 42(6): 554-556. DOI: 10.15932/j.0253-9713.2020.06.018. [8] Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会感染病学分会, 中华医学会肝病学分. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [9] BLEI AT, CÓRDOBA J. Hepatic encephalopathy[J]. Am J Gastroenterol, 2001, 96(7): 1968-1976. DOI: 10.1111/j.1572-0241.2001.03964.x. [10] CHANG ML, LIAW YF. Hepatitis B flares in chronic hepatitis B: Pathogenesis, natural course, and management[J]. J Hepatol, 2014, 61(6): 1407-1417. DOI: 10.1016/j.jhep.2014.08.033. [11] ANGELI P, GINÈS P, WONG F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites[J]. J Hepatol, 2015, 62(4): 968-974. DOI: 10.1016/j.jhep.2014.12.029. [12] Chinese Society of Spleen and Portal Hypertension Surgery, Chinese Society of Surgery, Chinese Medical Association. Expert consensus on diagnosis and treatment of esophagogastric variceal bleeding in cirrhotic portal hypertension (2019 edition)[J]. Chin J Surg, 2019, 57(12): 885-892. DOI: 10.3760/cma.j.issn.0529-5815.2019.12.002.中华医学会外科学分会脾及门静脉高压外科学组. 肝硬化门静脉高压症食管、胃底静脉曲张破裂出血诊治专家共识(2019版)[J]. 中华外科杂志, 2019, 57(12): 885-892. DOI: 10.3760/cma.j.issn.0529-5815.2019.12.002. [13] Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the management of ascites and complications in cirrhosis[J]. J Clin Hepatol, 2017, 33(10): 1847-1863. DOI: 10.3969/j.issn.1001-5256.2017.10.003.中华医学会肝病学分会. 肝硬化腹水及相关并发症的诊疗指南[J]. 临床肝胆病杂志, 2017, 33(10): 1847-1863. DOI: 10.3969/j.issn.1001-5256.2017.10.003. [14] Drug-induced Liver Disease Study Group, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the management of drug-induced liver injury[J]. J Clin Hepatol, 2015, 31(11): 1752-1769. DOI: 10.3969/j.issn.1001-5256.2015.11.002.中华医学会肝病学分会药物性肝病学组. 药物性肝损伤诊治指南[J]. 临床肝胆病杂志, 2015, 31(11): 1752-1769. DOI: 10.3969/j.issn.1001-5256.2015.11.002. [15] FERNANDO SM, ROCHWERG B, SEELY A. Clinical implications of the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)[J]. CMAJ, 2018, 190(36): E1058-E1059. DOI: 10.1503/cmaj.170149. [16] Chinese Society of Respirology, Chinese Medical Association. Guidelines for the diagnosis and treatment of community- acquired pneumonia in Chinese adults (2016 edition)[J]. Chin J Tuberc Respir Dis, 2016, 39(4): 253-279. DOI: 10.3760/cma.j.issn.1001-0939.2016.04.005.中华医学会呼吸病学分会. 中国成人社区获得性肺炎诊断和治疗指南(2016年版)[J]. 中华结核和呼吸杂志, 2016, 39(4): 253-279. DOI: 10.3760/cma.j.issn.1001-0939.2016.04.005. [17] KAMATH PS, WIESNER RH, MALINCHOC M, et al. A model to predict survival in patients with end-stage liver disease[J]. Hepatology, 2001, 33(2): 464-470. DOI: 10.1053/jhep.2001.22172. [18] LEE S, FARWELL AP. Euthyroid sick syndrome[J]. Compr Physiol, 2016, 6(2): 1071-1080. DOI: 10.1002/cphy.c150017. [19] PUNEKAR P, SHARMA AK, JAIN A. A study of thyroid dysfunction in cirrhosis of liver and correlation with severity of liver disease[J]. Indian J Endocrinol Metab, 2018, 22(5): 645-650. DOI: 10.4103/ijem.IJEM_25_18. [20] LI Q, WANG J, LU M, et al. Acute-on-chronic liver failure from chronic-hepatitis-B, who is the behind scenes[J]. Front Microbiol, 2020, 11: 583423. DOI: 10.3389/fmicb.2020.583423. [21] de LUCA R, DAVIS PJ, LIN HY, et al. Thyroid hormones interaction with immune response, inflammation and non-thyroidal illness syndrome[J]. Front Cell Dev Biol, 2020, 8: 614030. DOI: 10.3389/fcell.2020.614030. [22] CAI XJ, SHEN Y, ZHU XH, et al. Evaluation of integrated model for end-stage liver disease model in predicting prognosis of acute-on-chronic liver failure and choice of treatment[J/CD]. Chin J Exp Clin Infect Dis(Electronic Edition), 2018, 12(5): 446-452. DOI: 10.3877/cma.j.issn.1674-1358.2018.05.006.蔡晓娟, 沈毅, 朱晓红, 等. 整合终末期肝病模型在慢加急性肝功能衰竭预后判断及治疗中的应用[J/CD]. 中华实验和临床感染病杂志(电子版), 2018, 12(5): 446-452. DOI: 10.3877/cma.j.issn.1674-1358.2018.05.006. [23] ZHANG WJ, ZHAO LJ, WU JZ. Value of MELD、AARC、COSSH scoring systems in evaluating the 90-day prognosis of hepatitis B virus-related acute-on-chronic liver failure[J]. J Clin Hepatol, 2020, 36(4): 813-817. DOI: 10.3969/j.issn.1001-5256.2020.04.021.张文佳, 赵丽娟, 吴基洲. MELD、AARC、COSSH评分系统对乙型肝炎相关慢加急性肝衰竭90天预后的评估价值[J]. 临床肝胆病杂志, 2020, 36(4): 813-817. DOI: 10.3969/j.issn.1001-5256.2020.04.021. -



 PDF下载 ( 2175 KB)
PDF下载 ( 2175 KB)

 下载:
下载: