直接抗病毒药物治疗慢性丙型肝炎合并血小板减少患者的效果分析
DOI: 10.3969/j.issn.1001-5256.2022.01.014
Clinical effect of direct-acting antiviral agents in treatment of chronic hepatitis C patients with thrombocytopenia
-
摘要:
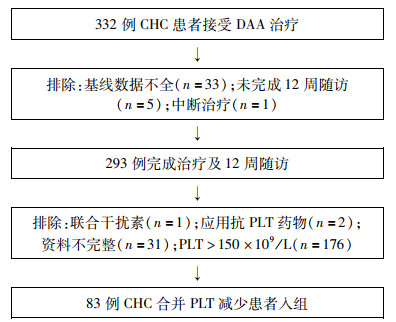
目的 探讨慢性丙型肝炎(CHC)合并血小板(PLT)减少患者应用直接抗病毒药物(DAA)的临床疗效及对PLT的影响。 方法 回顾分析2018年4月—2019年3月在天津市第三中心医院接受DAA治疗合并PLT减少(PLT<150×109/L)的CHC患者83例,应用无干扰素方案的DAA治疗12~24周,评估治疗结束(EOT)及结束后第12周患者病毒学应答、肝功能指标、PLT、肝硬度(LSM)的变化。正态分布的计量资料组间比较采用重复测量资料的方差分析。非正态分布的计量资料组间比较前进行正态转换,后行重复测量资料的方差分析。logistic回归分析影响PLT升高的预测因素。绘制受试者工作特征曲线(ROC曲线)评价基线LSM对治疗后PLT升高的预测价值。 结果 83例CHC合并PLT减少的患者中,肝硬化患者占61.4%,治疗结束后12周持续病毒学应答(SVR12)率为98.8%。与基线相比较,EOT及SVR12时,患者血清AST、ALT、GGT、TBil、Glo水平下降,Alb水平升高,LSM明显下降,差异均有统计学意义(P值均<0.05)。患者PLT在EOT[(110.4±44.6)×109/L]和SVR12[(109.0±47.7)×109/L]时均较基线[(97.8±33.2)×109/L]显著升高(P值均<0.01)。获得SVR12时,PLT升高与无升高组患者肝硬化比例、基线LSM及基线WBC水平差异均有统计学意义(P值均<0.05);多因素logistic回归分析表明,基线LSM是DAA治疗后PLT明显升高的独立预测因子(OR=0.929, 95%CI: 0.864~0.999, P<0.05)。基线LSM水平预测PLT升高的ROC曲线下面积为0.644,cut-off值为20.15 kPa,其灵敏度和特异度分别为81.0%和48.6%。基线PLT大于100×109/L组PLT升高幅度更明显(P<0.05)。 结论 CHC合并PLT减少患者在DAA治疗及获得SVR12后,肝功能、LSM有明显改善,基线LSM是PLT升高的独立预测因素,与基线相比较,PLT在EOT及SVR12有明显提升。 Abstract:Objective To investigate the clinical effect of direct-acting antiviral agent (DAA) in the treatment of chronic hepatitis C (CHC) patients with thrombocytopenia and its effect on platelet count (PLT). Methods A retrospective analysis was performed for 83 CHC patients with thrombocytopenia (PLT < 150×109/L) who received the DAA treatment regimen without interferon for 12-24 weeks in Tianjin Third Central Hospital from April 2018 to March 2019, and the changes in virologic response, liver function parameters, PLT, and liver stiffness measurement (LSM) were evaluated at the end of treatment (EOT) and at week 12 after EOT. Quantitative data accord with normal distribution were compared by repeated measures ANOVA. Normal transformation was performed before the comparison between skewed data, then repeated measures ANOVA was carried out. A logistic regression analysis was used to investigate the predictive factors for PLT elevation, and the receiver operating characteristic (ROC) curve was plotted to analyze the value of LSM in predicting PLT elevation after treatment. Results Among the 83 CHC patients with thrombocytopenia, 61.4% had liver cirrhosis, and the rate of sustained virologic response at week 12 after the end of treatment (SVR12) was 98.8%. From baseline to EOT and SVR12, the patients had significant reductions in the serum levels of aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transpeptidase, total bilirubin, and globin, a significant increase in the serum level of albumin, and a significant reduction in LSM (all P < 0.05). For all patients, PLT at EOT and SVR12 was significantly higher than that at baseline [EOT vs baseline: (110.4±44.6)×109/L vs (97.8±33.2)×109/L, P < 0.01; SVR12 vs baseline: (109.0±47.7)×109/L vs (97.8±33.2)×109/L, P < 0.01]. At SVR12, there were significant differences in the proportion of patients with liver cirrhosis, baseline LSM, and baseline white blood cell count between the PLT elevation group and the non-PLT elevation group (all P < 0.05). The multivariate logistic regression analysis showed that LSM was an independent predictive factor for significant PLT elevation after DAA treatment (odds ratio=0.929, 95% confidence interval: 0.864-0.999, P < 0.05). Baseline LSM had an area under the ROC curve of 0.644 in predicting PLT elevation, with a sensitivity of 81.0% and a specificity of 48.6% at a cut-off value of 20.15 kPa. The patients with PLT > 100×109/L at baseline had a greater increase in PLT(P < 0.05). Conclusion CHC patients with thrombocytopenia have significant improvements in liver function and LSM after receiving DAA treatment and obtaining SVR12, and baseline LSM is an independent predictive factor for PLT elevation. There is a significant increase in PLT from baseline to EOT and SVR12. -
Key words:
- Hepatitis C, Chronic /
- Antiviral Agents /
- Thrombocytopenia /
- Therapeutics
-
表 1 纳入83例患者的基线临床特征
项目 数值 BMI(kg/m2) 23.4±3.2 肝硬化基础[例(%)] 51(61.4) HCV RNA(log10IU/ml) 6.3(5.7~6.7) PLT(×109/L) 97.8±33.0 WBC(×109/L) 4.4(3.9~5.4) Cr(μmol/L) 64.0(53.0~73.0) Alb(g/L) 44.4(40.5~46.8) ALT(U/L) 41.0(26.0~82.3) AST(U/L) 51.5(32.8~75.3) TBil(μmol/L) 17.2(13.0~25.3) GGT(U/L) 48.0(26.0~72.0) Glo(g/L) 34.4(29.7~36.8) LSM(kPa) 15.5(11.1~23.6) APRI 1.4(0.8~2.5) 初治[例(%)] 68(81.9) HCV基因型[例(%)] 1b 68(81.9) 2a 12(14.5) 3a 2(2.4) 6a 1(1.2) 表 2 82例患者DAA治疗后肝功能、LSM、APPRI比较
项目 基线 EOT SVR12 AST(U/L) 53.0(33.0~76.5) 24.0(19.0~30.0)1) 24.0(20.0~31.0)1) ALT(U/L) 42.0(26.5~84.5) 18.0(14.0~26.3)1) 20.0(15.0~28.3)1) GGT(U/L) 47.5(25.8~73.8) 21.5(15.8~34.0)1) 21.0(15.5~30.0)1) Alb(g/L) 44.4(40.4~46.8) 46.8(43.8~49.3)1) 48.0(43.8~49.8)1) Glo(g/L) 34.1(29.7~36.8) 32.3(28.0~34.7)1) 31.4(28.3~33.5)1) TBil(μmol/L) 17.1(13.0~24.3) 16.8(11.6~22.4)1) 14.1(10.6~17.5)1) LSM(kPa) 15.2(11.0~22.8) 15.0(11.9~20.5)1) 13.1(9.9~17.4)1)2) APRI 1.40(0.85~2.53) 0.60(0.36~0.87)1) 0.60(0.39~1.04)1) 注:1)与基线比较,P<0.05;2)与EOT比较,P<0.01。 表 3 PLT升高组与无升高组基线特征对比
项目 PLT升高组(n=43) PLT无升高组(n=39) 统计值 P值 年龄(岁) 60.7±9.2 61.1±9.9 t=-0.479 0.632 男性[例(%)] 14(32.6) 15(38.5) χ2=0.312 0.577 肝硬化[例(%)] 21(48.8) 29(74.4) χ2=5.598 0.018 初治[例(%)] 6(14.0) 9(23.1) χ2=1.139 0.286 Alb(g/L) 44.7(42.2~47.4) 43.3(39.8~46.8) Z=-1.030 0.303 Glo(g/L) 33.4(29.5~36.3) 34.8(29.6~38.1) Z=-0.730 0.465 AST(U/L) 46(26~100) 57(35~69) Z=-0.383 0.702 ALT(U/L) 49.5(22.8~102.3) 39.0(29.0~72.0) Z=-0.662 0.508 ALP(U/L) 92(65~108) 86(70~99) Z=-0.430 0.667 GGT(U/L)) 48(29~85) 42(22~72) Z=-1.109 0.267 TBil(μmol/L) 16.9(13.1~27.0) 17.2(12.0~23.8) Z=-0.076 0.940 AFP(ng/mL) 6.0(3.8~13.6) 6.7(3.2~14.6) Z=-0.306 0.759 APRI 1.30(0.70~2.80) 1.41(1.04~1.91) Z=-0.482 0.630 LSM(kPa) 13.6(10.4~19.7) 19.2(11.6~26.3) Z=-2.191 0.028 HCV RNA(log10IU/mL) 6.4(5.7~6.8) 6.1(5.7~6.5) Z=-1.393 0.164 WBC(×109/L) 4.8(4.1~5.8) 4.2(3.5~4.9) Z=-1.996 0.046 PLT(×109/L) 99(79~136) 93(67~115) Z=-1.458 0.145 HGB(g/L) 138(128~152) 136(127~149) Z=-0.683 0.495 Cr(μmol/L) 64(52~77) 64(53~71) Z=-0.245 0.806 表 4 logistic回归分析PLT升高的预测因素
参数 OR 95%CI P值 基线肝硬化 0.440 0.126~1.533 0.197 基线WBC 1.225 0.809~1.853 0.337 基线PLT 0.989 0.970~1.009 0.275 基线LSM 0.929 0.864~0.999 0.046 基线ALT 1.004 0.995~1.013 0.378 基线GGT 1.012 0.997~1.028 0.122 -
[1] WEDEMEYER H, DORE GJ, WARD JW. Estimates on HCV disease burden worldwide - filling the gaps[J]. J Viral Hepat, 2015, 22(Suppl 1): 1-5. DOI: 10.1111/jvh.12371. [2] GONZALEZ HC, DUARTE-ROJO A. Virologic cure of hepatitis C: Impact on hepatic fibrosis and patient outcomes[J]. Curr Gastroenterol Rep, 2016, 18(7): 32. DOI: 10.1007/s11894-016-0508-y. [3] LOUIE KS, MICALLEF JM, PIMENTA JM, et al. Prevalence of thrombocytopenia among patients with chronic hepatitis C: A systematic review[J]. J Viral Hepat, 2011, 18(1): 1-7. DOI: 10.1111/j.1365-2893.2010.01366.x. [4] KEE KM, WANG JH, HUNG CH, et al. Improvement of thrombocytopenia in hepatitis C-related advanced fibrosis patients after sustained virological response[J]. Dig Dis Sci, 2013, 58(2): 556-561. DOI: 10.1007/s10620-012-2380-4. [5] van der MEER AJ, MAAN R, VELDT BJ, et al. Improvement of platelets after SVR among patients with chronic HCV infection and advanced hepatic fibrosis[J]. J Gastroenterol Hepatol, 2016, 31(6): 1168-1176. DOI: 10.1111/jgh.13252. [6] Chinese Society of Hepatology, Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for hepatitis C: A 2015 update[J]. J Clin Hepatol, 2015, 31(12): 1961-1979. DOI: 10.3969/j.issn.1001-5256.2015.12.003.中华医学会肝病学分会, 中华医学会感染病学会分会. 丙型肝炎防治指南(2015年更新版)[J]. 临床肝胆病杂志, 2015, 31(12): 1961-1979. DOI: 10.3969/j.issn.1001-5256.2015.12.003. [7] European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018[J]. J Hepatol, 2018, 69(2): 461-511. DOI: 10.1016/j.jhep.2018.03.026. [8] Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35(11): 2408-2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006.中华医学会肝病学分会. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35(11): 2408-2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. [9] HUI P, COOK DJ, LIM W, et al. The frequency and clinical significance of thrombocytopenia complicating critical illness: A systematic review[J]. Chest, 2011, 139(2): 271-278. DOI: 10.1378/chest.10-2243. [10] CHEN YC, TSENG CW, TSENG KC. Rapid platelet count improvement in chronic hepatitis C patients with thrombocytopenia receiving direct-acting antiviral agents[J]. Medicine (Baltimore), 2020, 99(19): e20156. DOI: 10.1097/MD.0000000000020156. [11] DIETRICH CF, BAMBER J, BERZIGOTTI A, et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (long version)[J]. Ultraschall Med, 2017, 38(4): e48. DOI: 10.1055/a-0641-0076. [12] Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of hepatitis C(2019 version)[J]. J Clin Hepatol, 2019, 35(12): 2670-2686. DOI: 10.3969/j.issn.1001-5256.2019.12.008.中华医学会肝病学分会, 中华医学会感染病学分会. 丙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2670-2686. DOI: 10.3969/j.issn.1001-5256.2019.12.008. [13] EL-SERAG HB, CHRISTIE IC, PUENPATOM A, et al. The effects of sustained virological response to direct-acting anti-viral therapy on the risk of extrahepatic manifestations of hepatitis C infection[J]. Aliment Pharmacol Ther, 2019, 49(11): 1442-1447. DOI: 10.1111/apt.15240. [14] HERMOS JA, QUACH L, GAGNON DR, et al. Incident severe thrombocytopenia in veterans treated with pegylated interferon plus ribavirin for chronic hepatitis C infection[J]. Pharmacoepidemiol Drug Saf, 2014, 23(5): 480-488. DOI: 10.1002/pds.3585. [15] HUANG CF, YEH ML, HUANG CI, et al. Interference of hepatitis B virus dual infection in platelet count recovery in chronic hepatitis C patients with curative antiviral therapy[J]. J Gastroenterol Hepatol, 2018, 33(5): 1108-1114. DOI: 10.1111/jgh.14017. [16] LOUIE KS, MICALLEF JM, PIMENTA JM, et al. Prevalence of thrombocytopenia among patients with chronic hepatitis C: A systematic review[J]. J Viral Hepat, 2011, 18(1): 1-7. DOI: 10.1111/j.1365-2893.2010.01366.x. [17] RAWI S, WU GY. Pathogenesis of thrombocytopenia in chronic HCV infection: A review[J]. J Clin Transl Hepatol, 2020, 8(2): 184-191. DOI: 10.14218/JCTH.2020.00007. [18] OSADA M, KANEKO M, SAKAMOTO M, et al. Causes of thrombocytopenia in chronic hepatitis C viral infection[J]. Clin Appl Thromb Hemost, 2012, 18(3): 272-280. DOI: 10.1177/1076029611429124. [19] AREF S, SLEEM T, EL MENSHAWY N, et al. Antiplatelet antibodies contribute to thrombocytopenia associated with chronic hepatitis C virus infection[J]. Hematology, 2009, 14(5): 277-281. DOI: 10.1179/102453309X439818. [20] OGAWA E, FURUSYO N, NAKAMUTA M, et al. Glecaprevir and pibrentasvir for Japanese patients with chronic hepatitis C genotype 1 or 2 infection: Results from a multicenter, real-world cohort study[J]. Hepatol Res, 2019, 49(6): 617-626. DOI: 10.1111/hepr.13328. [21] SEKO Y, MORIGUCHI M, TAKAHASHI A, et al. The association between the platelet count and liver volume in compensated cirrhosis patients after the eradication of hepatitis C virus by direct-acting antivirals[J]. Intern Med, 2020, 59(15): 1811-1817. DOI: 10.2169/internalmedicine.4442-20. [22] DAI CY, HO CK, HUANG JF, et al. Hepatitis C virus viremia and low platelet count: A study in a hepatitis B & C endemic area in Taiwan[J]. J Hepatol, 2010, 52(2): 160-166. DOI: 10.1016/j.jhep.2009.11.017. [23] KAJIHARA M, KATO S, OKAZAKI Y, et al. A role of autoantibody-mediated platelet destruction in thrombocytopenia in patients with cirrhosis[J]. Hepatology, 2003, 37(6): 1267-1276. DOI: 10.1053/jhep.2003.50209. [24] HONMA Y, SHIBATA M, HAYASHI T, et al. Effect of direct-acting antivirals on platelet-associated immunoglobulin G and thrombocytopenia in hepatitis C virus-related chronic liver disease[J]. Liver Int, 2019, 39(9): 1641-1651. DOI: 10.1053/jhep.2003.50209. [25] SHAO LN, ZHANG ST, WANG N, et al. Platelet indices significantly correlate with liver fibrosis in HCV-infected patients[J]. PLoS One, 2020, 15(1): e0227544. DOI: 10.1371/journal.pone.0227544. [26] GOTLIEB N, SCHWARTZ N, ZELBER-SAGI S, et al. Longitudinal decrease in platelet counts as a surrogate marker of liver fibrosis[J]. World J Gastroenterol, 2020, 26(38): 5849-5862. DOI: 10.3748/wjg.v26.i38.5849. [27] KODA M, MATUNAGA Y, KAWAKAMI M, et al. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C[J]. Hepatology, 2007, 45(2): 297-306. DOI: 10.1002/hep.21520. [28] COVERDALE SA, SAMARASINGHE DA, LIN R, et al. Changes in antipyrine clearance and platelet count, but not conventional liver tests, correlate with fibrotic change in chronic hepatitis C: Value for predicting fibrotic progression[J]. Am J Gastroenterol, 2003, 98(6): 1384-1390. DOI: 10.1111/j.1572-0241.2003.07468.x. [29] TANIGUCHI H, IWASAKI Y, FUJIWARA A, et al. Long-term monitoring of platelet count, as a non-invasive marker of hepatic fibrosis progression and/or regression in patients with chronic hepatitis C after interferon therapy[J]. J Gastroenterol Hepatol, 2006, 21(1 Pt 2): 281-287. DOI: 10.1111/j.1440-1746.2006.04201.x. [30] CHANG Y, KIM JI, LEE B, et al. Clinical application of ultrasonography-guided percutaneous liver biopsy and its safety over 18 years[J]. Clin Mol Hepatol, 2020, 26(3): 318-327. DOI: 10.3350/cmh.2019.0019n. [31] NAKAGOMI R, TATEISHI R, MASUZAKI R, et al. Liver stiffness measurements in chronic hepatitis C: Treatment evaluation and risk assessment[J]. J Gastroenterol Hepatol, 2019, 34(5): 921-928. DOI: 10.1111/jgh.14530. [32] WANG JH, CHANGCHIEN CS, HUNG CH, et al. Liver stiffness decrease after effective antiviral therapy in patients with chronic hepatitis C: Longitudinal study using FibroScan[J]. J Gastroenterol Hepatol, 2010, 25(5): 964-969. DOI: 10.1111/j.1440-1746.2009.06194.x. [33] ABDEL ALEM S, ELSHARKAWY A, EL AKEL W, et al. Liver stiffness measurements and FIB-4 are predictors of response to sofosbuvir-based treatment regimens in 7256 chronic HCV patients[J]. Expert Rev Gastroenterol Hepatol, 2019, 13(10): 1009-1016. DOI: 10.1080/17474124.2019.1653183. -



 PDF下载 ( 2173 KB)
PDF下载 ( 2173 KB)

 下载:
下载:



