基于SEER数据的原发性小肝癌肝切除术后生存率列线图模型的构建及验证
DOI: 10.3969/j.issn.1001-5256.2022.01.017
Establishment of a nomogram for survival rate after liver resection for primary small hepatocellular carcinoma based on SEER data and external validation
-
摘要:
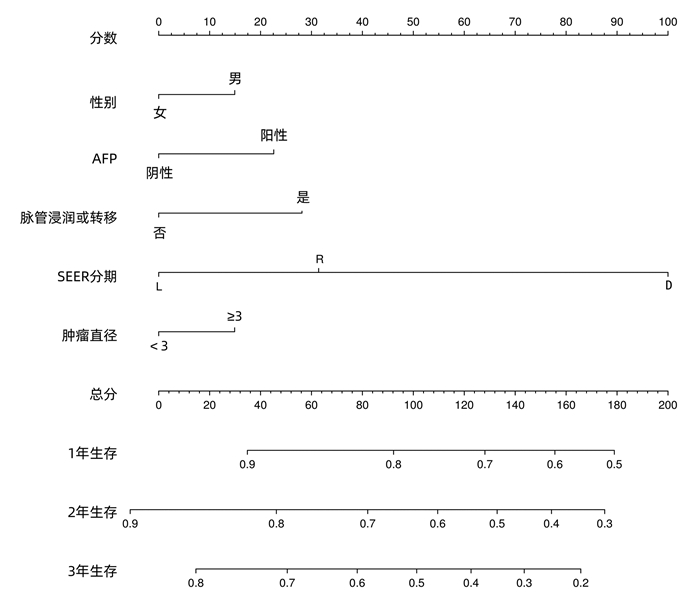
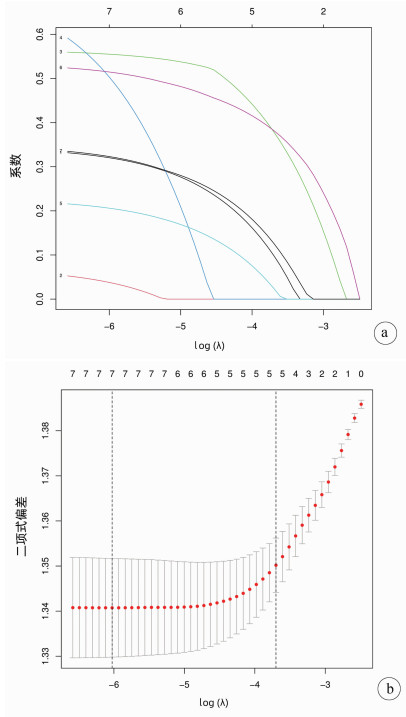
目的 基于SEER数据和中国数据建立并验证原发性小肝癌肝切除术后总生存率列线图模型。 方法 提取2004年—2015年美国国立癌症研究所SEER数据库所登记的原发性小肝癌接受肝切除术治疗的1809例患者资料作为建模组;收集2010年—2017年在川北医学院附属医院接受肝切除术治疗的小肝癌患者158例为验证组。采用单因素Cox风险回归、lasso回归、多因素Cox风险回归分析小肝癌患者肝切除术后OS的影响因素。根据OS的独立影响因素构建列线图模型,利用一致性指数(C-index)、绘制校准曲线及ROC曲线检验模型的预测能力。利用Kaplan-Meier生存分析和log-rank检验分析高、低风险组间的生存差异。 结果 多因素Cox风险回归分析发现性别(HR=1.22, 95%CI:1.05~1.41, P=0.010)、Seer分期(HR=1.51, 95%CI:1.23~1.85, P<0.001;HR=10.31, 95%CI:2.53~42.04, P=0.001)、肿瘤直径(HR=1.22,95%CI:1.06~1.39,P=0.004)、脉管侵犯或转移(HR=1.43,95%CI:1.24~1.65,P<0.001)、AFP(HR=1.33,95%CI:1.16~1.54,P<0.001)是小肝癌患者肝切除术后OS的独立危险因素。建模组C指数为0.621,其1、2、3年ROC曲线下面积分别为0.666(95%CI:0.628~0.704),0.678(95%CI:0.647~0.708),0.663(95%CI:0.635~0.690);验证组C指数为0.718;其1、2、3年ROC曲线下面积分别为0.695(95%CI:0.593~0.797),0.781(95%CI:0.706~0.856),0.759(95%CI:0.669~0.848)。根据列线图模型进行风险分层,Kaplan-Meier生存分析显示:建模组和验证组的低风险组的预后均明显优于高风险组(P<0.01)。 结论 建立的原发性小肝癌肝切除术后生存率模型可以用于预测1、2、3年OS,可以使用于国内临床工作。 Abstract:Objective To establish a nomogram for overall survival rate after liver resection for primary small hepatocellular carcinoma based on SEER data and external validation of Chinese data. Methods The data of 1809 patients, registered in National Cancer Institute SEER database in 2004-2015, who underwent hepatectomy for primary small hepatocellular carcinoma were extracted as modeling group, and 158 patients with small hepatocellular carcinoma who underwent hepatectomy in Affiliated Hospital of North Sichuan Medical College from 2010 to 2017 were collected as validation group. The univariate Cox risk regression analysis, lasso regression analysis, and multivariate Cox hazard regression analysis were used to investigate the influencing factors for OS after hepatectomy in patients with small hepatocellular carcinoma. A nomogram was established based on the independent influencing factors for OS, and index of concordance (C-index), calibration curves, and receiver operating characteristic (ROC) curve were used to analyze the predictive ability of the nomogram. The Kaplan-Meier survival analysis and the log-rank test were used to investigate the difference in survival between the high- and low-risk groups. Results The multivariate Cox hazard regression analysis showed that sex (hazard ratio [HR]=1.22, 95% confidence interval [CI]: 1.05-1.41, P=0.010), Seer stage (HR=1.51, 95%CI: 1.23-1.85, P < 0.001; HR=10.31, 95%CI: 2.53-42.04, P=0.001), tumor diameter (HR=1.22, 95%CI: 1.06-1.39, P=0.004), vascular invasion or metastasis (HR=1.43, 95%CI: 1.24-1.65, P < 0.001), and alpha-fetoprotein (HR=1.33, 95%CI: 1.16-1.54, P < 0.001) were independent risk factors for OS after hepatectomy for small hepatocellular carcinoma. The modeling group had a C-index of 0.621, and its area under the ROC curve at 1, 2, and 3 years was 0.666(95%CI 0.628-0.704), 0.678(95%CI 0.647-0.708), and 0.663(95%CI: 0.635-0.690), respectively; the validation group had a C-index of 0.718, and its area under the ROC curve at 1, 2, and 3 years was 0.695(95%CI: 0.593-0.797), 0.781(95%CI: 0.706-0.856), and 0.759(95%CI 0.669-0.848), respectively. Risk stratification was performed based on the nomogram, and the Kaplan-Meier survival analysis showed that for both the modeling group and the validation group, the low-risk group had a significantly better prognosis than the high-risk group (P < 0.01). Conclusion The model established for survival rate after liver resection for primary small hepatocellular carcinoma can predict the 1-, 2-, and 3-year OS rates and can thus be used in clinical practice in China. -
Key words:
- Carcinoma, Hepatocellular /
- Hepatectomy /
- Nomograms
-
表 1 建模组和验证组一般资料
指标 建模组(n=1809) 验证组(n=158) 年龄[例(%)] <60岁 695(38.4) 114(72.2) ≥60岁 1114(61.6) 44(27.8) 性别[例(%)] 女 527(29.1) 26(16.5) 男 1282(70.9) 132(83.5) 分化程度[例(%)] 高 368(20.3) 9(5.7) 中 991(54.8) 89(56.3) 低/未 450(24.9) 60(38.0) Seer分期[例(%)] L 1613(89.2) 139(88.0) R 179(9.9) 18(11.4) D 17(0.9) 1(0.6) N分期[例(%)] N0 1799(99.4) 152(96.2) N1 10(0.6) 6(3.8) M分期[例(%)] M0 1794(99.2) 157(99.4) M1 15(0.8) 1(0.6) 肿瘤直径[例(%)] <3 cm 724(40.0) 46(29.1) ≥3 cm 1085(60.0) 112(70.9) 脉管侵犯或转移[例(%)] 无 1185(65.5) 115(72.8) 有 624(34.5) 43(27.2) AFP[例(%)] 阴性 698(38.6) 75(47.5) 阳性 1111(61.4) 83(52.5) 肿瘤个数[例(%)] 单发 1369(75.7) 139(88.0) 多发 440(24.3) 19(12.0) 注:AFP<20 ng/ml为阴性。 表 2 建模组OS的单因素及多因素Cox分析结果
变量 单因素分析 多因素分析 HR 95%CI P值 HR 95%CI P值 年龄 <60岁 ≥60岁 1.13 0.99~1.30 0.066 性别 女 男 1.19 1.03~1.38 0.022 1.22 1.05~1.41 0.010 分化程度 高 中 0.82 0.70~0.97 0.018 低/未 0.76 0.63~0.92 0.005 Seer分期 L R 1.97 1.63~2.38 <0.001 1.51 1.23~1.85 <0.001 D 3.98 2.39~6.65 <0.001 10.31 2.53~42.04 0.001 N分期 N0 N1 1.43 0.68~3.02 0.342 M分期 M0 M1 3.35 1.93~5.80 <0.001 肿瘤直径 <3 cm ≥3 cm 1.25 1.09~1.42 0.001 1.22 1.06~1.39 0.004 脉管侵犯或转移 无 有 1.68 1.48~1.92 <0.001 1.43 1.24~1.65 <0.001 AFP 阴性 阳性 1.40 1.22~1.60 <0.001 1.33 1.16~1.54 <0.001 肿瘤个数 单发 多发 1.01 0.87~1.18 0.866 -
[1] TORRE LA, BRAY F, SIEGEL RL, et al. Global cancer statistics, 2012[J]. CA Cancer J Clin, 2015, 65(2): 87-108. DOI: 10.3322/caac.21262. [2] WEN T, JIN C, FACCIORUSSO A, et al. Multidisciplinary management of recurrent and metastatic hepatocellular carcinoma after resection: An international expert consensus[J]. Hepatobiliary Surg Nutr, 2018, 7(5): 353-371. DOI: 10.21037/hbsn.2018.08.01. [3] AHN KS, KANG KJ. Appropriate treatment modality for solitary small hepatocellular carcinoma: Radiofrequency ablation vs. resection vs. transplantation?[J]. Clin Mol Hepatol, 2019, 25(4): 354-359. DOI: 10.3350/cmh.2018.0096. [4] LI CH, HU P, OU YJ, et al. Development and validation of nomogram for predicting survival outcomes in patients with small hepatocellular carcinoma[J]. J Third Mil Med Univ, 2020, 42(20): 2046-2056. DOI: 10.16016/j.1000-5404.202007005.李传红, 胡鹏, 欧晏娇, 等. 预测小肝癌患者生存率列线图的建立和验证[J]. 第三军医大学学报, 2020, 42(20): 2046-2056. DOI: 10.16016/j.1000-5404.202007005. [5] ZHANG W, WANG X, JIANG R, et al. Effect of tumor size on cancer-specific survival in small hepatocellular carcinoma[J]. Mayo Clin Proc, 2015, 90(9): 1187-1195. DOI: 10.1016/j.mayocp.2015.06.018. [6] WEI Y, DAI F, YI Y, et al. Impact of local tumor lesion treatments and preoperative indicators on the survival of patients with small hepatocellular carcinomas[J]. Oncol Lett, 2018, 16(4): 5050-5058. DOI: 10.3892/ol.2018.9263. [7] VOLK ML. Unfair priority for HCC: A problem whose ideal solution remains unsolved[J]. Am J Transplant, 2010, 10(7): 1507-1508. DOI: 10.1111/j.1600-6143.2010.03154.x. [8] ZANG YY, LONG PY, HAN ZW, et al. Establishment and verification of a nomogram for predicting patient prognosis after hepatectomy for hepatocellular carcinoma[J]. J Clin Hepatol, 2019, 35(10): 2225-2230. DOI: 10.3969/j.issn.1001-5256.2019.10.019.臧游亚, 龙沛雲, 韩志伟, 等. 肝细胞癌患者肝切除术后预后列线图的建立与验证[J]. 临床肝胆病杂志, 2019, 35(10): 2225-2230. DOI: 10.3969/j.issn.1001-5256.2019.10.019. [9] SALVATORE M, JEON J, MEZA R. Changing trends in liver cancer incidence by race/ethnicity and sex in the US: 1992-2016[J]. Cancer Causes Control, 2019, 30(12): 1377-1388. DOI: 10.1007/s10552-019-01237-4. [10] BALACHANDRAN VP, GONEN M, SMITH JJ, et al. Nomograms in oncology: More than meets the eye[J]. Lancet Oncol, 2015, 16(4): e173-e180. DOI: 10.1016/S1470-2045(14)71116-7. [11] JADLOWIEC CC, TANER T. Liver transplantation: Current status and challenges[J]. World J Gastroenterol, 2016, 22(18): 4438-4445. DOI: 10.3748/wjg.v22.i18.4438. [12] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI: 10.3322/caac.21660. [13] AN L, ZENG HM, ZHENG RS, et al. Liver cancer epidemiology in China, 2015[J]. Chin J Oncol, 2019, 41(10): 721-722. DOI: 10.3760/cma.j.issn.0253-3766.2019.10.001.安澜, 曾红梅, 郑荣寿, 等. 2015年中国肝癌流行情况分析[J]. 中华肿瘤杂志, 2019, 41(10): 721-722. DOI: 10.3760/cma.j.issn.0253-3766.2019.10.001. [14] CHEN K, HUO RR, CHEN SY, et al. Analysis of the relationship between gender and prognosis of patients after liver resection for hepatocellular carcinoma[J]. Chin J Hepatobiliary Surg, 2020, 26(5): 330-334. DOI: 10.3760/cma.j.cn113884-20190909-00290.陈康, 霍荣瑞, 陈肃伊, 等. 性别对肝细胞癌患者肝切除术后预后的影响[J]. 中华肝胆外科杂志, 2020, 26(5): 330-334. DOI: 10.3760/cma.j.cn113884-20190909-00290. [15] GALLE PR, FOERSTER F, KUDO M, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma[J]. Liver Int, 2019, 39(12): 2214-2229. DOI: 10.1111/liv.14223. [16] ZHU Y, XIA QQ, LUO J, et al. Application of combined detection of alpha-fetoprotein, alpha-fetoprotein heterogeneity and TNF-α factor in the diagnosis of primary liver cancer and its clinical value[J]. J Clin Exp Med, 2020, 19(4): 383-386. DOI: 10.3969/j.issn.1671-4695.2020.04.014.朱艳, 夏芹芹, 罗俊, 等. 甲胎蛋白、甲胎蛋白异质体与TNF-α因子联合检测在原发性肝癌诊断中的应用价值[J]. 临床和实验医学杂志, 2020, 19(4): 383-386. DOI: 10.3969/j.issn.1671-4695.2020.04.014. [17] GRAHAM JA, MELANCON JK, SHETTY K, et al. Liver transplantation should be offered to patients with small solitary hepatocellular carcinoma and a positive serum alpha fetoprotein rather than resection[J]. Am J Surg, 2013, 205(4): 374-380. DOI: 10.1016/j.amjsurg.2012.12.002. [18] TANG H, TANG XY, LIU M, et al. Targeting alpha-fetoprotein represses the proliferation of hepatoma cells via regulation of the cell cycle[J]. Clin Chim Acta, 2008, 394(1-2): 81-88. DOI: 10.1016/j.cca.2008.04.012. [19] LU Y, ZHU M, LI W, et al. Alpha fetoprotein plays a critical role in promoting metastasis of hepatocellular carcinoma cells[J]. J Cell Mol Med, 2016, 20(3): 549-558. DOI: 10.1111/jcmm.12745. [20] ZHANG XP, CHEN ZH, ZHOU TF, et al. A nomogram to predict early postoperative recurrence of hepatocellular carcinoma with portal vein tumour thrombus after R0 liver resection: A large-scale, multicenter study[J]. Eur J Surg Oncol, 2019, 45(9): 1644-1651. DOI: 10.1016/j.ejso.2019.03.043. [21] WANG Y, SUN K, SHEN J, et al. Novel prognostic nomograms based on inflammation-related markers for patients with hepatocellular carcinoma underwent hepatectomy[J]. Cancer Res Treat, 2019, 51(4): 1464-1478. DOI: 10.4143/crt.2018.657. [22] YUAN SX, ZHOU WP. Progress and hot spots of comprehensive treatment for primary liver cancer[J]. Chin J Dig Surg, 2021, 20(2) : 163-170. DOI: 10.3760/cma.j.cn115610-20201211-00776.袁声贤, 周伟平. 原发性肝癌综合治疗的进展和热点[J]. 中华消化外科杂志, 2021, 20(2): 163-170. DOI: 10.3760/cma.j.cn115610-20201211-00776. [23] LU XY, XI T, LAU WY, et al. Pathobiological features of small hepatocellular carcinoma: Correlation between tumor size and biological behavior[J]. J Cancer Res Clin Oncol, 2011, 137(4): 567-575. DOI: 10.1007/s00432-010-0909-5. [24] MINAGAWA M, IKAI I, MATSUYAMA Y, et al. Staging of hepatocellular carcinoma: Assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13, 772 patients in Japan[J]. Ann Surg, 2007, 245(6): 909-922. DOI: 10.1097/01.sla.0000254368.65878.da. [25] ZHOU DD, LIU XL, JIANG TT, et al. Analysis and comparison of common prognostic prediction models for liver cancer[J]. Chin J Clin Oncol, 2020, 47(24): 1281-1286. DOI: 10.3969/j.issn.1000-8179.2020.24.149.周冬冬, 刘晓利, 姜婷婷, 等. 常见肝癌预后预测模型的分析与比较[J]. 中国肿瘤临床, 2020, 47(24): 1281-1286. DOI: 10.3969/j.issn.1000-8179.2020.24.149. [26] WEN L, WENG S, YAN C, et al. A radiomics nomogram for preoperative prediction of early recurrence of small hepatocellular carcinoma after surgical resection or radiofrequency ablation[J]. Front Oncol, 2021, 11: 657039. DOI: 10.3389/fonc.2021.657039. [27] ZHOU ZW, LIU YC, XIANG BD, et al. Global outlook for primary liver cancer: Prevalence, risk, factors and population attribution scores[J]. Chin J Oncol Prev Treat, 2021, 13(1): 14-21. DOI: 10.3969/j.issn.1674-5671.2021.01.03.周泽文, 刘颖春, 向邦德, 等. 原发性肝癌的全球展望: 流行情况、危险因素和人群归因分值[J]. 中国癌症防治杂志, 2021, 13(1): 14-21. DOI: 10.3969/j.issn.1674-5671.2021.01.03. -



 PDF下载 ( 4273 KB)
PDF下载 ( 4273 KB)

 下载:
下载: