GDC-0449在CCl4联合二乙酰氨基芴诱导肝纤维化大鼠模型中的作用
DOI: 10.3969/j.issn.1001-5256.2022.02.016
Role of GDC-0449 in a rat model of liver fibrosis induced by carbon tetrachloride combined with 2-acetylaminofluorene
-
摘要:
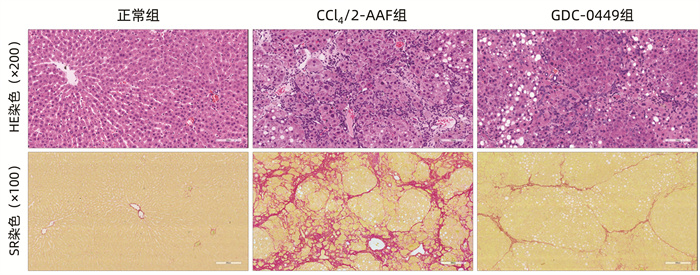
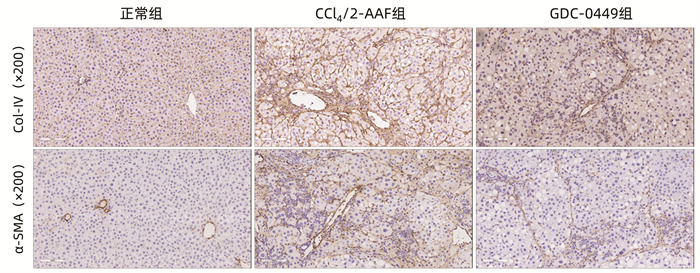
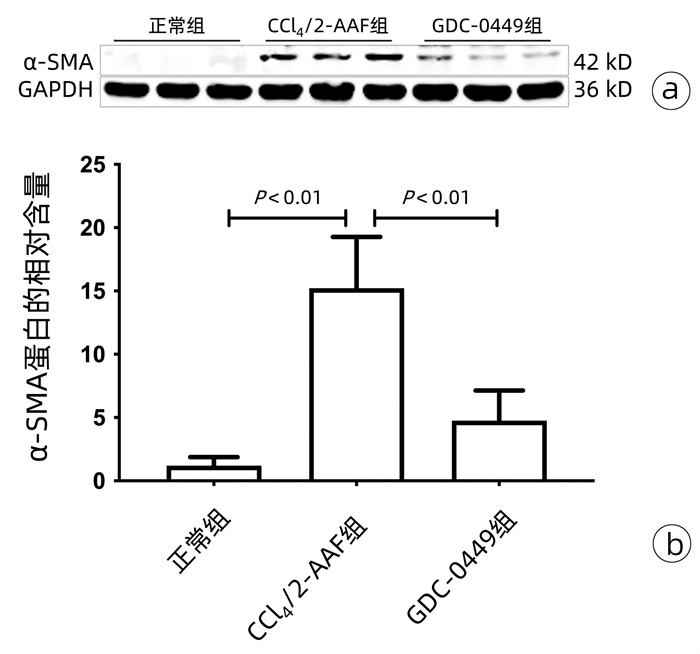
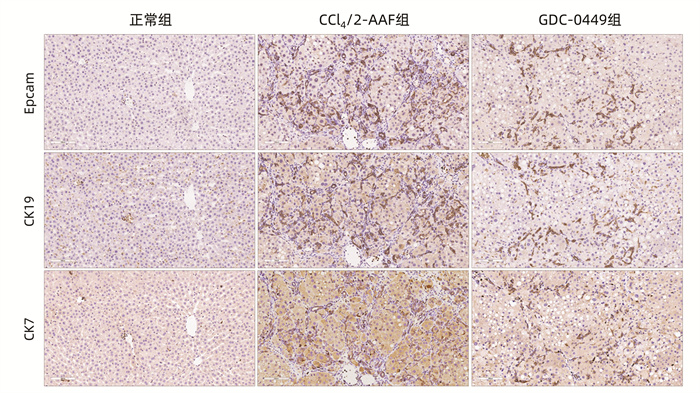
目的 研究Hedgehog信号通路抑制剂维莫德吉(GDC-0449)对CCl4联合二乙酰氨基芴(2-AAF)诱导的大鼠肝纤维化的干预作用。 方法 将18只Fisher344雌性大鼠随机分为正常组、CCl4联合2-AAF模型组(CCl4/2-AAF组)和GDC-0449组,每组6只。CCl4/2-AAF组和GDC-0449组大鼠按2 mL/kg体质量皮下注射30% CCl4-橄榄油溶液,每周2次,持续造模6周;自第7周始,在注射CCl4-橄榄油溶液同时予2-AAF(100 mg·kg-1·d-1)灌胃,同时GDC-0449组大鼠按25 mg·kg-1·d-1灌胃干预,正常组大鼠予等体积橄榄油溶液注射及生理盐水灌胃,9周末处死取材。HE染色和天狼猩红(SR)染色观察各组大鼠肝组织病理及胶原沉积变化,SR阳性染色面积半定量分析和Ishak评分评估纤维化程度;碱水解法测定肝组织羟脯氨酸(Hyp)水平;免疫组化、Western Blot、实时荧光定量聚合酶链式反应(qRT-PCR)检测肝组织α平滑肌肌动蛋白(α-SMA)、Ⅰ型胶原(Col-Ⅰ)、Ⅳ型胶原(Col-Ⅳ)、细胞角蛋白(CK)19、CK7、上皮细胞黏附分子(Epcam)及Hedgehog信号通路表达变化;免疫荧光共染观察CK19和卵圆细胞标志物6(OV6)共定位情况。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 与正常组相比,CCl4/2-AAF组大鼠肝组织炎性细胞聚集、胶原纤维沉积明显,存在明显的假小叶结构;肝组织Hyp水平和胶原阳性面积比显著增加(P值均<0.05),Ishak评分显著上升(P<0.05);α-SMA、Col-Ⅰ、Col-Ⅳ、Epcam、CK19、CK7、跨膜转运蛋白Smoothened(Smo)、Hedgehog配体Desert Hedgehog(Dhh)、Indian Hedgehog结合膜受体Patched(Ptch2)、胶质瘤相关致癌基因(Gli1、Gli2、Gli3)的表达显著增多(P值均<0.05);免疫荧光共染显示CCl4/2-AAF组大鼠肝组织内CK19阳性细胞均表达OV6,且较正常组显著增多。与CCl4/2-AAF组相比,GDC-0449组肝组织炎性细胞聚集、胶原沉积明显减少;肝组织Hyp水平、胶原阳性面积比显著降低(P值均<0.05),Ishak评分显著下降(P<0.05);α-SMA、Epcam、CK19、CK7、Smo、Ptch2、Gli1、Gli2和Gli3的表达显著减少(P值均<0.05);免疫荧光共染显示肝组织内共表达OV6与CK19的阳性细胞数显著减少。 结论 Hedgehog信号通路抑制剂GDC-0449显著抑制了CCl4/2-AAF诱导的大鼠肝纤维化进展,且其作用机制与抑制肝星状细胞活化、胶原沉积,阻抑肝祖细胞活化增殖,并抑制其向胆管上皮细胞分化有关。 -
关键词:
- 肝硬化 /
- Hedgehog信号通路 /
- 大鼠, 近交F344
Abstract:Objective To investigate the intervention effect of GDC-0449, a hedgehog signaling pathway inhibitor, on rats with liver fibrosis induced by carbon tetrachloride (CCl4) combined with 2-acetylaminofluorene (2-AAF). Methods A total of 18 female Fisher344 rats were randomly divided into normal group, CCl4/2-AAF group, and GDC-0449 group, with 6 rats in each group. The rats in the CCl4/2-AAF group and the GDC-0449 group were given subcutaneously injected 30% CCl4-olive oil solution at a dose of 2 mL/kg twice a week for 6 weeks to induce liver fibrosis; since week 7, in addition to the injection of CCl4-olive oil solution, the rats in these two groups were given 2-AAF (100 mg/kg/d) by gavage, and the rats in the GDC-0449 group were given GDC-0449 (25 mg/kg/d) by gavage, while those in the normal group were given an equal volume of olive oil solution by injection and normal saline by gavage. All rats were sacrificed at the end of week 9, and related samples were collected. HE staining and sirius red (SR) staining were used to observe the changes in liver histopathology and collagen deposition, and the semi-quantitative analysis of SR-positive area and Ishak score were used to evaluate fibrosis degree; the alkaline hydrolysis method was used to measure the level of hydroxyproline (Hyp) in liver tissue; immunohistochemistry, Western blot, and qRT-PCR were used to measure the expression of α-smooth muscle actin (α-SMA), type Ⅰ collagen (Col-Ⅰ), type Ⅳ collagen (Col-Ⅳ), cytokeratin 19 (CK19), cytokeratin 7 (CK7), the epithelial cell adhesion molecule Epcam, and the hedgehog signaling pathway in liver tissue; double immunofluorescence staining was used to observe the colocalization of CK19 and the oval cell marker OV6. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results Compared with the normal group, the CCl4/2-AAF group had marked inflammatory cell aggregation and collagen deposition in liver tissue, with the formation of a pseudolobular structure, as well as significant increases in Hyp level and collagen positive area ratio in liver tissue (P < 0.05), Ishak score (P < 0.05), and the expression of α-SMA, Col-Ⅰ, Col-Ⅳ, Epcam, CK19, CK7, the transmembrane transporter Smoothened (Smo), Hedgehog ligand Desert Hedgehog (Dhh), the Indian Hedgehog membrane-binding receptor Patched (Ptch2), and glioma-related oncogenes Gli1, Gli2, and Gli3 (all P < 0.05); double immunofluorescence staining showed that CK19-positive cells also expressed OV6 in the liver tissue of rats in the CCl4/2-AAF group, with a significant increase compared with the normal group. Compared with the CCl4/2-AAF group, the GDC-0449 group had significant reductions in inflammatory cell aggregation and collagen deposition in liver tissue, Hyp level and collagen positive area ratio in liver tissue (P < 0.05), Ishak score (P < 0.05), and the expression of α-SMA, Epcam, CK19, CK7, Smo, Ptch2, Gli1, Gli2, and Gli3 (all P < 0.05); double immunofluorescence staining showed a significant reduction in the number of cells with co-expression of OV6 and CK19 in liver tissue. Conclusion The Hedgehog signaling pathway inhibitor GDC-0449 can significantly inhibit the progression of liver fibrosis induced by CCl4/2-AAF in rats, possibly by inhibiting hepatic stellate cell activation, collagen deposition, activation and proliferation of hepatic progenitor cells, and differentiation of hepatic progenitor cells into biliary epithelial cells. -
Key words:
- Liver Cirrhosis /
- Hedgehog Signaling Pathway /
- Rats, Inbred F344
-
原发性胆汁性胆管炎(primary biliary cholangitis,PBC)旧称原发性胆汁性肝硬化,是一种慢性进行性胆汁淤积性肝病,其组织学特点是肝内小叶间胆管非化脓性肉芽肿性炎症,引起小叶间胆管消失,最终导致胆汁性肝纤维化,甚至胆汁性肝硬化和肝衰竭[1-2]。PBC的血清学特征是出现抗线粒体抗体(anti-mitochondrial antibody, AMA)2型。大部分的PBC患者为40岁以上的中老年女性,其中1/3的患者无任何症状,通常在常规体检中被发现。从出现症状时起,平均生存期为5~8年,早期诊治对预后起着关键作用[3-5]。目前治疗PBC的主要药物为熊去氧胆酸(ursodeoxycholic acid,UDCA),经大量的临床研究证实,UDCA可明显改善多数PBC患者的肝功能,有效延缓病情进展从而延长生存时间。但是仍有约25%~40%的PBC患者对UDCA应答不佳[6],部分应答不佳的患者,根据需要改用或者加用二线治疗药物如奥贝胆酸、免疫抑制剂或布地奈德等则可以取得良好的疗效[7-10]。在评估PBC患者对UDCA等药物的应答情况方面,肝组织病理学比血清生化学更能准确地反应出用药以来的病情变化[11]。
传统的PBC组织学分期有Rubin分期、Scheuer分期和Ludwig分期,近年又有Nakanuma分期。虽然按新指南要求,PBC确诊不再完全依赖病理,但基于PBC的胆管损伤具有典型的形态学特点,特别对于AMA阴性PBC,肝活检确诊仍有重要意义,且肝组织病理更能准确反映PBC进展[12]。此外,PBC治疗效果评价、新药靶点的发现和选择,亦要依赖肝组织病理。现对不同PBC的组织学分期进行综述,为临床医师从事PBC诊治及相关研究提供参考。
1. 病理学分期系统
1.1 Rubin分期系统
Rubin等[13]在1965年提出PBC早期的特点是汇管区炎细胞浸润和小叶间胆管损伤、小叶间胆管周围淋巴细胞和浆细胞的聚集,通常无明显胆汁淤积;中期出现胆管的增生和破坏,可见小叶中心和周围肝细胞胆汁淤积;晚期表现为肝硬化。
1.2 Scheuer分期系统
Scheuer[14]在1967年将PBC分为4期:Ⅰ期的组织学改变为胆管损伤;Ⅱ期的组织学改变主要是胆管增生;Ⅲ期出现了桥接瘢痕,纤维隔;Ⅳ期为肝硬化。Hans Popper和Schaffner将Scheuer分期系统进行修订[15]:Ⅰ期为胆管明显损伤(汇管区炎症);Ⅱ期为细胆管增生(汇管区周围扩张和炎症);Ⅲ期为桥接瘢痕,纤维隔;Ⅳ期为肝硬化。
1.3 Ludwig分期系统
1978年Ludwig提出了PBC分期系统[15],Ⅰ期组织学改变为汇管区炎症;Ⅱ期组织学改变为汇管区周围炎,即界面炎;Ⅲ期出现桥接坏死、纤维化;Ⅳ期为肝硬化。
1.4 Nakanuma分期系统
Nakanuma在2010年提出了一个新的分期系统[16],根据纤维化、胆管缺失、地衣红阳性颗粒沉积和坏死性炎症活动的严重程度进行分期和分级(表 1)。(1)对于纤维化,0分指的是几乎没有纤维化或纤维化局限于汇管区;1分指的是纤维化范围超出汇管区,偶有不完全纤维间隔;2分为桥接纤维化伴小叶紊乱;3分为肝硬化(广泛纤维化和再生结节)。(2)对于胆管缺失,0分指的是标本中的所有汇管区均可见小叶间胆管;1分和2分分别指的是在<1/3和1/3~ 2/3的汇管区有胆管缺失;3分指的是>2/3的汇管区有胆管缺失。(3)地衣红阳性颗粒是肝细胞溶酶体中的铜结合蛋白,它们的沉积反映了慢性胆盐淤积的程度,0分指的是汇管区周围肝细胞无沉积;1分指的是<1/3的汇管区周围肝细胞有沉积;3分为>2/3的汇管区或纤维间隔周围肝细胞有沉积;介于以上两种表现之间为2分。(4)在对每一项进行评分后,获得总分,根据总分进行分期。Ⅰ期(无进展):总分为0分;Ⅱ期(轻度进展):总分为1~3分;Ⅲ期(中度进展):总分为4~6分;Ⅳ期(重度进展):总分为7~9分。如果无法进行地衣红染色,则纤维化和胆管缺失评分的总和也适用,Ⅰ期(无进展):总分为0分;Ⅱ期(轻度进展):总分为1~2分;Ⅲ期(中度进展):总分为3~4分;Ⅳ期(重度进展):总分为5~6分。
表 1 Nakanuma评分项目 病理表现 纤维化评分 0分 无纤维化,或纤维化局限于汇管区 1分 汇管区纤维化伴汇管区周围纤维化或不完全纤维间隔 2分 桥接纤维化伴小叶紊乱 3分 肝硬化伴再生结节和广泛纤维化 胆管缺失评分 0分 无胆管缺失 1分 <1/3的汇管区有胆管缺失 2分 1/3~2/3的汇管区有胆管缺失 3分 >2/3的汇管区有胆管缺失 地衣红阳性颗粒的沉积评分 0分 无地衣红阳性颗粒沉积 1分 <1/3的汇管区周围肝细胞有颗粒沉积 2分 1/3~2/3的汇管区周围肝细胞有颗粒沉积 3分 >2/3的汇管区周围肝细胞有颗粒沉积 坏死性炎症活动分级,分为胆管炎活动(cholangitis activity,CA)和肝炎活动(hepatitis activity,HA),这两种病变是PBC病理的两种重要改变,活动性胆管炎分级为CA 0~3,活动性肝炎分级为HA 0~3。CA0(无活动):无胆管炎,但可能存在轻度胆管上皮细胞损伤;CA1(轻度活动):可见一个受损的胆管表现为明显的慢性胆管炎(受损的胆管完全被轻度-中度的淋巴-浆细胞包围)。但是小叶间胆管被少量淋巴-浆细胞浸润或邻近淋巴细胞浸润的汇管区,则不被视为明显的慢性胆管炎;CA2(中度活动):超过两个胆管伴有明显的慢性胆管炎;CA3(重度活动):至少有一个受损的胆管表现为慢性非化脓性胆管炎,慢性非化脓性胆管炎表现为胆管上皮细胞受损,胆管完全被大量的淋巴-浆细胞浸润,受损的胆管部分或全部被上皮样肉芽肿包围(肉芽肿性胆管炎)。活动性肝炎(HA)分为4个等级,HA0(无活动):无界面肝炎,且无或最轻微的小叶性肝炎;HA1(轻度活动):指界面肝炎累及一个汇管区或纤维间隔周围连续10个肝细胞,且有轻度-中度小叶性肝炎;HA2(中度活动):界面肝炎累及两个以上汇管区或纤维间隔周围连续10个肝细胞,且有轻度-中度小叶性肝炎;HA3(重度活动):界面肝炎累及超过一半数量的汇管区周围连续20个肝细胞,且有中度小叶性肝炎或桥接或带状坏死。
2. 讨论与总结
1950年“原发性胆汁性肝硬化”命名正式确定。随着对PBC诊断认识的深入,1965年Rubin及Hans Popper通过对大量PBC肝活检病例系统总结,阐述了PBC的形态学特点,尤为侧重PBC的形态学诊断及鉴别诊断,并对病变演变规律做了最初的总结。Scheuer系统,特别是Hans Popper修订版,较为清晰的提出4期分期方案,这一方案在应用和验证中获得很好的一致性。Ludwig明确界定各分期之间的分界点,包括Ⅰ期强调炎症限于汇管区间质,Ⅱ期强调了汇管区周围炎,即界面炎的重要性,Hans Popper修订版强调的是细胆管反应增生,Ludwig认为Ⅲ期出现桥接坏死或桥接纤维化,并强调了它们是PBC晚期(胆汁性肝硬化)的主要原因,这一点是继Hans Popper修订版之后的重大发现。
传统分期体现了病理学家对PBC病变特点和进展规律的认识不断成熟的历程,尤其Ludwig分期系统,至今仍被美国肝病学会和欧洲肝病学会作为指南推荐,广泛应用于PBC相关的临床及科研,其在PBC病理学发展过程中具有不可取代的位置[17-18]。
虽然经典PBC分期方法各有自身优势,但随着这些分期系统在临床上被广泛应用,各自的局限性也渐渐显现。Rubin分期较为笼统不够精确,肝硬化阶段被清楚地描述,但肝硬化前各阶段之间的区别并不清楚[19]。Scheuer分期的缺点是各个分期阶段有大量的重叠,如Ⅳ期的患者肝脏可呈现Ⅰ或Ⅱ期的胆管改变。Popper和Schaffner与Scheuer主要不同的观点在于前者认为Ⅰ期的汇管区炎症不会累及肝实质,而Scheuer却有着相反的观点。在Ludwig的系统强调了胆管损伤不同阶段对于肝实质的影响,病变早期限于汇管区间质内,继而出现界面炎,最后形成胆汁性肝纤维化甚至胆汁性肝硬化。然而,非常重要的胆管损伤程度和胆汁淤积变化却未被评估在内。
现阶段,由于临床诊断PBC的水平提高,PBC诊断和分期已不再完全依赖病理活检,病理技术需要被赋予新的历史任务和目标,如预测疾病进展、寻找新的/有效的治疗靶点和评估疗效的指标等,这样就需要涵盖更多敏感有效指标的新评价系统的出现,因此Nakanuma分期和分级系统应运而生。Nakanuma系统通过对肝纤维化、胆管缺失、地衣红阳性颗粒的沉积进行评分,再进行分期,并联合坏死性炎症活动的分级综合评判患者的严重程度与预后。
与传统分期系统相比,Nakanuma分期系统与肝硬化相关症状的发展具有更好的相关性,有研究[20]报道坏死性炎症程度的分级与血清学指标如ALT、AST、ALP、GGT、TBil、DBil、IgM、IgG呈正相关。有研究[19]在同一组PBC病例10年随访中发现,以3种不同系统分期,患者出现肝硬化相关症状的比例不同。以Nakanuma系统划分,则Ⅰ~Ⅳ期患者随访10年分别有0、12.6%、40.6%和100%的病例进展至肝硬化;以Scheuer系统划分,则Ⅰ~Ⅲ期患者随访10年分别有7.5%、100%和40.3%的病例进展至肝硬化;以Ludwig系统划分,则Ⅰ~Ⅲ期患者随访10年分别有17.6%、4.6%和47.4%的病例进展至肝硬化。Nakanuma系统分期的肝硬化发生率随着分期的升高而升高。在随访中,对Nakanuma分期系统的组织学研究,观察到地衣红阳性颗粒的沉积与肝纤维化呈显著相关,胆管缺失评分为2或3分,特别是在地衣红阳性颗粒沉积评分为2~3分的患者中,肝硬化相关疾病的发展速度明显高于评分为0~1分的患者。因此与经典分期相比,Nakanuma分期系统更准确地预测了PBC患者的10年预后情况,尤其是肝硬化及其并发症。Nakanuma分期系统的Ⅰ期患者随访中并未出现肝硬化,Ⅱ期和Ⅲ期之间肝硬化的发生率有显著性差异。然而,在Scheuer系统和Ludwig系统中未观察到这种趋势。
在取样过程中,典型的病变可能被遗漏在活检样本之外,增加了假阴性的几率,Nakanuma分期利用评分系统,更合理地最大程度地减少PBC肝组织学固有的采样误差,提供给临床医生更多客观信息,可常规应用于PBC组织学评估。目前,该分期系统广泛应用仍存在待研究的问题,例如,系统涉及评价项目繁琐,病理医生操作不易,一致性可能欠满意等。其次,新旧系统的对比研究论文比较少,需要更大队列验证,以更准确地验证这种新的Nakanuma分级和分期系统。旧系统在当下及未来仍具有举足轻重的作用,新旧系统可结合使用,从而为PBC患者的合理防治提供指导。
-
表 1 各组大鼠肝组织SR阳性染色面积比、Hyp水平及Ishak评分比较
组别 动物数(只) SR阳性染色面积比(%) Hyp(μg/mL) Ishak评分(分) 正常组 6 1.05±0.37 335.63±51.75 0.00±0.00 CCl4/2-AAF组 6 12.18±3.901) 1 003.18±297.331) 13.17±1.721) GDC-0449组 6 3.99±1.432) 645.54±69.602) 8.50±1.382) F值 28.69 17.45 164.80 P值 <0.001 <0.001 <0.001 注:与正常组比较,1)P<0.05;与CCl4/2-AAF组比较,2)P<0.05。 表 2 各组大鼠肝组织Col-Ⅰ、Col-Ⅳ、Acta2 mRNA表达量变化
组别 动物数(只) Col-ⅠmRNA Col-Ⅳ mRNA Acta2 mRNA 正常组 6 0.59±0.29 0.78±0.23 1.82±0.66 CCl4/2-AAF组 6 21.73±9.401) 6.56±2.021) 9.10±2.431) GDC-0449组 6 17.34±2.19 4.80±1.43 5.18±0.972) F值 19.99 21.16 27.47 P值 <0.001 <0.001 <0.001 注:与正常组比较,1)P<0.05;与CCl4/2-AAF组比较,2)P<0.05。 表 3 各组大鼠肝组织Epcam、CK19、CK7和Ccnd1 mRNA表达水平
组别 动物数(只) Epcam mRNA CK19 mRNA CK7 mRNA Ccnd1 mRNA 正常组 6 1.32±0.42 0.57±0.27 1.00±0.18 1.14±0.15 CCl4/2-AAF组 6 7.59±1.951) 8.64±1.031) 1.71±0.271) 2.30±0.161) GDC-0449组 6 3.07±1.492) 5.47±1.812) 1.18±0.242) 1.91±0.302) F值 25.26 56.28 12.07 38.21 P值 <0.001 <0.001 0.002 <0.001 注:与正常组比较,1)P<0.05;与CCl4/2-AAF组比较,2)P<0.05。 表 4 各组大鼠肝组织Hedgehog信号通路相关因子mRNA表达量变化
组别 动物数(只) Smo mRNA Dhh mRNA Ptch2 mRNA Gli1 mRNA Gli2 mRNA Gli3 mRNA 正常组 6 0.87±0.09 1.10±0.29 0.62±0.27 0.80±0.24 0.65±0.24 0.85±0.27 CCl4/2-AAF组 6 4.31±1.301) 12.89±2.791) 3.20±0.861) 10.00±3.651) 1.62±0.391) 1.65±0.341) GDC-0449组 6 2.60±0.352) 10.14±4.53 0.82±0.222) 0.48±0.262) 0.96±0.032) 0.98±0.132) F值 24.45 20.11 36.00 32.59 17.35 13.42 P值 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 注:与正常组比较,1)P<0.05;与CCl4/2-AAF组比较,2)P<0.05。 -
[1] LLOVET JM, ZUCMAN-ROSSI J, PIKARSKY E, et al. Hepatocellular carcinoma[J]. Nat Rev Dis Primers, 2016, 2: 16018. DOI: 10.1038/nrdp.2016.18. [2] D'AMICO G, MORABITO A, D'AMICO M, et al. New concepts on the clinical course and stratification of compensated and decompensated cirrhosis[J]. Hepatol Int, 2018, 12(Suppl 1): 34-43. DOI: 10.1007/s12072-017-9808-z. [3] PAROLA M, PINZANI M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues[J]. Mol Aspects Med, 2019, 65: 37-55. DOI: 10.1016/j.mam.2018.09.002. [4] ASRANI SK, DEVARBHAVI H, EATON J, et al. Burden of liver diseases in the world[J]. J Hepatol, 2019, 70(1): 151-171. DOI: 10.1016/j.jhep.2018.09.014. [5] HERNANDEZ-GEA V, FRIEDMAN SL. Pathogenesis of liver fibrosis[J]. Annu Rev Pathol, 2011, 6: 425-456. DOI: 10.1146/annurev-pathol-011110-130246. [6] KISSELEVA T, BRENNER D. Molecular and cellular mechanisms of liver fibrosis and its regression[J]. Nat Rev Gastroenterol Hepatol, 2021, 18(3): 151-166. DOI: 10.1038/s41575-020-00372-7. [7] MARCELLIN P, GANE E, BUTI M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study[J]. Lancet, 2013, 381(9865): 468-475. DOI: 10.1016/S0140-6736(12)61425-1. [8] D'AMBROSIO R, AGHEMO A, RUMI MG, et al. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis[J]. Hepatology, 2012, 56(2): 532-543. DOI: 10.1002/hep.25606. [9] VILAR-GOMEZ E, MARTINEZ-PEREZ Y, CALZADILLA-BERTOT L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis[J]. Gastroenterology, 2015, 149(2): 367-378. DOI: 10.1053/j.gastro.2015.04.005. [10] MACHADO MV, DIEHL AM. Hedgehog signalling in liver pathophysiology[J]. J Hepatol, 2018, 68(3): 550-562. DOI: 10.1016/j.jhep.2017.10.017. [11] SHACKEL NA, MCGUINNESS PH, ABBOTT CA, et al. Identification of novel molecules and pathogenic pathways in primary biliary cirrhosis: cDNA array analysis of intrahepatic differential gene expression[J]. Gut, 2001, 49(4): 565-576. DOI: 10.1136/gut.49.4.565. [12] ISHAK K, BAPTISTA A, BIANCHI L, et al. Histological grading and staging of chronic hepatitis[J]. J Hepatol, 1995, 22(6): 696-699. DOI: 10.1016/0168-8278(95)80226-6. [13] LO RC, KIM H. Histopathological evaluation of liver fibrosis and cirrhosis regression[J]. Clin Mol Hepatol, 2017, 23(4): 302-307. DOI: 10.3350/cmh.2017.0078. [14] OMENETTI A, CHOI S, MICHELOTTI G, et al. Hedgehog signaling in the liver[J]. J Hepatol, 2011, 54(2): 366-373. DOI: 10.1016/j.jhep.2010.10.003. [15] FARGNOLI MC, PELLEGRINI C, PICCERILLO A, et al. Clinical determinants of complete response to vismodegib in locally advanced basal cell carcinoma: A multicentre experience[J]. J Eur Acad Dermatol Venereol, 2021, 35(12): e923-e926. DOI: 10.1111/jdv.17588. [16] PRATAP A, SINGH S, MUNDRA V, et al. Attenuation of early liver fibrosis by pharmacological inhibition of smoothened receptor signaling[J]. J Drug Target, 2012, 20(9): 770-782. DOI: 10.3109/1061186X.2012.719900. [17] SICKLICK JK, LI YX, CHOI SS, et al. Role for hedgehog signaling in hepatic stellate cell activation and viability[J]. Lab Invest, 2005, 85(11): 1368-1380. DOI: 10.1038/labinvest.3700349. [18] KUMAR V, DONG Y, KUMAR V, et al. The use of micelles to deliver potential hedgehog pathway inhibitor for the treatment of liver fibrosis[J]. Theranostics, 2019, 9(25): 7537-7555. DOI: 10.7150/thno.38913. [19] SICKLICK JK, LI YX, MELHEM A, et al. Hedgehog signaling maintains resident hepatic progenitors throughout life[J]. Am J Physiol Gastrointest Liver Physiol, 2006, 290(5): g859-g870. DOI: 10.1152/ajpgi.00456.2005. [20] OMENETTI A, SYN WK, JUNG Y, et al. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production[J]. Hepatology, 2009, 50(2): 518-527. DOI: 10.1002/hep.23019. [21] OMENETTI A, POPOV Y, JUNG Y, et al. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats[J]. Gut, 2008, 57(9): 1275-1282. DOI: 10.1136/gut.2008.148619. [22] OMENETTI A, YANG L, LI YX, et al. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation[J]. Lab Invest, 2007, 87(5): 499-514. DOI: 10.1038/labinvest.3700537. 期刊类型引用(7)
1. 朱金霞,刘光伟,杨培伟. 中医药干预自身免疫性肝炎Th17/Treg平衡的研究进展. 中西医结合肝病杂志. 2023(04): 378-381 .  百度学术
百度学术2. 余钰娟,毛德文,刘茵,周晓云. 单味中药治疗自身免疫性肝炎的研究进展. 中国现代医生. 2023(30): 120-122+125 .  百度学术
百度学术3. 杨新荣,窦霞,李国峰,宋沁洁,李咸慰,吴红伟,李东辉,李越峰. 柴胡皂苷D对肝病防治作用及机制研究进展. 中草药. 2022(12): 3852-3862 .  百度学术
百度学术4. 李枝锦,吴平财,周山. 调肝运脾化浊方联合电针治疗非酒精性脂肪肝. 长春中医药大学学报. 2021(01): 103-107 .  百度学术
百度学术5. 郝健亨,李振城,刘莹,侯艺文,高艳,苗宇船,刘杨,郝慧琴. 差异表达microRNA参与刀豆蛋白A诱导的自身免疫性肝炎小鼠模型肝损伤的功能分析. 临床肝胆病杂志. 2021(06): 1360-1367 .  本站查看
本站查看6. 王旭红,李耀辉,张军城,李哲,刘改霞,张涛,张暮盈. 基于网络药理学的柴胡在小柴胡汤中治疗发热的作用分析. 海南医学院学报. 2021(16): 1262-1267 .  百度学术
百度学术7. 张晓莹,王雄文. 柴胡及柴胡剂的“推陈致新”理论探析. 长春中医药大学学报. 2020(06): 1097-1099 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 6040 KB)
PDF下载 ( 6040 KB)

 下载:
下载:
 下载:
下载:

 百度学术
百度学术

