扶正化瘀方通过改变急性肝损伤小鼠模型肝脏CD8+T淋巴细胞表型功能预防肝纤维化的价值分析
DOI: 10.3969/j.issn.1001-5256.2022.02.017
Value of Fuzheng Huayu prescription in preventing liver fibrosis by altering the phenotypic function of CD8+ T lymphocytes in the liver of mice with acute liver injury
-
摘要:
目的 观察应用扶正化瘀方后急性肝损伤小鼠模型肝脏CD8+ T淋巴细胞对共培养的肝星状细胞的影响,探讨扶正化瘀方预防肝纤维化的作用机制。 方法 18只SPF级雄性C57BL/6NCrl Vr小鼠随机分为正常组、模型组、扶正化瘀方组,每组6只。扶正化瘀方组提前给予扶正化瘀方灌胃5 d。实验前12 h以2 mL/kg体质量的剂量腹腔注射10%的CCl4,腹主静脉取血,留取血清并检测ALT、AST,留取部分肝脏做病理观察;应用流式细胞术分选小鼠肝脏CD8+ T淋巴细胞,预先小鼠体内用药,再与小鼠肝星状细胞株(JS 1)以2∶ 1比例共培养于96孔板中24 h、48 h,使用qPCR方法检测各组Col.Ⅰ mRNA和α-SMA mRNA表达变化。符合正态分布的计量资料多组间比较采用方差分析,进一步两两比较采用SNK-q或LSD-t检验。 结果 模型组的ALT和AST水平均显著高于正常组(P值均<0.000 1)。扶正化瘀方组小鼠的ALT升高的幅度明显小于模型组(P<0.001)。HE染色显示,扶正化瘀方组小鼠肝细胞变性、坏死程度较模型组有所减轻。与正常组比较, 模型组总淋巴细胞、CD45、CD4-CD8+T、CD8+CD28-T显著上升, 而扶正化瘀方组与模型组相比较, 上述淋巴细胞比例显著降低(P值均<0.001)。自各组小鼠肝脏分离的CD8+ T淋巴细胞与JS 1共培养48 h,模型组与对照组(JS 1单培养)和正常组相比α-SMA mRNA的表达明显增加(P值均<0.01);Col.Ⅰ mRNA表达也明显高于对照组和正常组(正常小鼠肝脏CD8+ T淋巴细胞与JS 1共培养)(P值均<0.001)。扶正化瘀方组α-SMA mRNA和Col.Ⅰ mRNA表达显著低于模型组(P值均<0.01)。 结论 扶正化瘀方能通过改变小鼠肝脏CD8+ T淋巴细胞功能表型间接抑制活化的肝星状细胞。 -
关键词:
- 肝硬化 /
- 扶正化瘀方 /
- 肝星状细胞 /
- CD8阳性T淋巴细胞
Abstract:Objective To investigate the effect of liver CD8+ T lymphocytes on co-cultured hepatic stellate cells (HSCs) after the application of Fuzheng Huayu prescription in a moues model of acute liver injury, as well as the mechanism of action of Fuzheng Huayu prescription in preventing liver fibrosis. Methods A total of 18 specific pathogen-free male C57BL/6NCrl Vr mice were randomly divided into normal group, model group, and Fuzheng Huayu prescription group, with 6 mice in each group. The mice in the Fuzheng Huayu prescription group were given Fuzheng Huayu prescription for 5 days in advance. At 12 hours before the experiment, 10% CCl4 was injected intraperitoneally at a dose of 2 mL/kg body weight. Blood was collected from the main abdominal vein, and the serum was separated to measure the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Part of the liver was used for pathological observation. After the mice were pretreated with medication in vivo, flow cytometry was used for the sorting of mouse liver CD8+ T lymphocytes, which were then co-cultured with the mouse HSC cell line (JS 1) in a 96-well plate at a ratio of 2∶ 1, and after co-culture for 24 and 48 hours, qPCR was used to measure the changes in the mRNA expression of Col.I and α-SMA. An analysis of variance was used for comparison of normally distributed continuous data between multiple groups, and the SNK-q test or the least significant difference t-test was used for further comparison between two groups. Results The model group had significantly higher activities of ALT and AST than the normal group (both P < 0.000 1), and compared with the model group, the Fuzheng Huayu prescription group had a significantly lower degree of increase in ALT activity (P < 0.001). HE staining showed that the Fuzheng Huayu prescription group had a significantly lower degree of hepatocyte degeneration and necrosis compared with the model group. Compared with the normal group, the total lymphocytes, CD45, CD4-CD8+ T and CD8 + CD28-T in the model group increased significantly, while the proportion of the above lymphocytes in the Fuzheng Huayu formula group decreased significantly compared with the model group (P < 0.001). CD8+ T lymphocytes isolated from the liver of mice in each group were co-cultured with JS 1 for 48 hours, and compared with the control group (JS 1 cultured alone) and the normal group, the model group had a significant increase in the mRNA expression of α-SMA (both P < 0.01) and significantly higher mRNA expression of Col.I than the control group and the normal group (normal mouse liver CD8+ T lymphocytes co-cultured with JS 1) (both P < 0.001). The Fuzheng Huayu prescription group had significantly lower mRNA expression levels of α-SMA and Col.I than the model group (both P < 0.01). Conclusion Fuzheng Huayu prescription can indirectly inhibit activated HSCs by altering the functional phenotype of CD8+ T lymphocytes in mouse liver. -
肝纤维化是许多慢性肝病,包括慢性乙型肝炎和丙型肝炎、酒精滥用、胆道梗阻、自身免疫性疾病、遗传性血液色素沉着症和非酒精性脂肪性肝病等[1]伴随的病理变化。肝纤维化最终可导致肝硬化、肝功能衰竭和肝癌。为了降低肝硬化和肝癌的病死率, 逆转肝纤维化是十分必要的[2]。对肝纤维化的治疗与研究在现阶段仍处于十分重要的地位。
肝星状细胞(HSC)的激活是肝纤维化产生的中心事件。肝纤维化是伤口愈合过程异常的原因,导致HSC不断活化,肝脏瘢痕组织形成。而免疫系统特别是T淋巴细胞、NK细胞通过细胞因子参与了HSC的活化和凋亡的调控。其中T淋巴细胞介导的免疫应答涉及CD8细胞毒性T淋巴细胞, 主要通过主要组织相容性复合体Ⅰ类分子识别感染细胞上的病毒抗原[3-4]。许多针对HBV的治疗性疫苗和免疫治疗策略, 主要集中于如何诱导适应性免疫应答,尤其是涉及特定T淋巴细胞的适应性免疫应答,因此,确定HBV特异性效应CD8+T淋巴细胞对HBV感染控制的影响至关重要。为了更确切地揭示扶正化瘀方抗肝纤维化的免疫调节机制,本文研究经扶正化瘀方干预后小鼠肝脏CD8+ T淋巴细胞对HSC的调节作用。
1. 材料与方法
1.1 实验动物
7周龄SPF级雄性C57BL/6NCrl Vr小鼠,购自北京维通利华动物有限公司[生产许可证:SCXK(京)2016-0011],饲养于上海中医药大学实验动物中心,实验动物使用许可证号:SYXK(沪)2014-0008。予以普通饮食饮水。
1.2 药物与试剂
四氯化碳(CCl4)和橄榄油均购自国药集团化学试剂有限公司。扶正化瘀方干粉,批号:180308,由上海现代中医药股份有限公司提供。将分析纯级别的CCl4与橄榄油按照1∶ 9比例混合,配制成浓度为10%的CCl4混合溶液。RPMI-1640,Hyclone;高糖DMEM培养基(1×),Corning;胎牛血清(FBS),Corning;PercollTM,GE Healthcare公司;Purified Rat Anti-Mouse CD16/CD32 (Mouse BD Fc BlockTM)、FITC Hamster Anti-Mouse CD3e、BV421 Rat Anti-Mouse CD45、PerCP-CyTM5.5 Rat Anti-Mouse CD4、APC Rat Anti-Mouse CD8a、PE-CF594 Hamster Anti-Mouse CD28,均购自BD公司;Cell to cDNA Kit PLUS、2×SYBR Green qPCR Mix,EZ Bioscience公司。BSA,上海碧云天生物技术有限公司。根据相关文献[5]制备扶正化瘀方药物血清。
1.3 主要仪器
生物安全柜,青岛海尔特种电器有限公司;细胞培养箱,Thermo Fisher Scientific公司;小型高速冷冻离心机,德国Eppendorf公司;低温高速离心机,JOUAN公司;全自动细胞计数仪、细胞计数板、cDNA逆转录仪,BIO-RAD公司;PCR扩增仪,ABI公司;流式细胞分选仪(型号:BD FACSAriaTM Cell Sorter),BD公司。
1.4 造模
参照文献报道,对模型小鼠给予10%的CCl4腹腔注射12 h,制备急性肝损伤模型。
1.5 分组及干预
动物实验:将18只C57雄性小鼠随机分为正常组、模型组、扶正化瘀方组,每组6只。适应性喂养1周。扶正化瘀方组提前给予扶正化瘀方灌胃5 d。实验开始前1天晚上给予模型组和扶正化瘀方组小鼠浓度为10%的CCl4,以2 mL/kg体质量的剂量腹腔注射。染毒12 h后,2%戊巴比妥麻醉,腹主静脉取血,无菌条件下,摘取肝脏用于病理观察和淋巴细胞分选。之后行流式细胞分选术。小鼠肝星状细胞株(JS 1)以10%血清培养,之后与CD8+T淋巴细胞以2∶ 1比例培养于96孔板中,重复3批。
1.6 检测指标及方法
(1) 使用微板法检测小鼠血清中ALT、AST含量。(2)将小鼠肝组织在10%中性甲醛中浸泡固定48 h后逐级酒精脱水,二甲苯透明,56 ℃石蜡包埋,4 μm厚切片,用于HE染色,光镜下观察。(3)qPCR检测JS 1中纤维状胶原蛋白Ⅰ(Collagen type Ⅰ,Col.Ⅰ) mRNA和α-平滑肌肌动蛋白(α-SMA) mRNA的表达(表 1)。按照EZ BioscienceTM qPCR SYBR Green Master Mix Protocol试剂盒进行qPCR检测实验。(4)使用美国BD公司流式细胞仪(BD FACSAriaTM Cell Sorter)无菌分选小鼠肝脏中CD8+T淋巴细胞。
表 1 qPCR引物列表Cat.No. TM值 名称 上游引物 下游引物 Tnt0338 85.1 GAPDH 5′AAA TGG TGA AGG TCG GTG TG 3′ 5′AGG TCA ATG AAG GGG TCG TT 3′ Tnt1328 81.1 α-SMA 5′CAC ACA CCT TTT AGA ACC CA 3′ 5′GTT TTC CCC CTT CCT CTT ACT 3′ Tnt1680a 85.9 Col.Ⅰ 5′TGA CTG GAA GAG CGG AGA GTA 3′ 5′GAC GGC TGA GTA GGG AAC AC 3′ 1.7 伦理学审查
本研究方案经由上海中医药大学实验动物伦理委员会审批,伦理审查号:PZSHUTCM190301014,符合实验室动物管理与使用准则。
1.8 统计学方法
采用SPSS 25.0统计软件进行数据分析。符合正态分布的计量资料用x±s表示,多组间比较采用单因素方差分析,进一步两两比较采用SNK-q或LSD-t检验。P<0.05为差异有统计学意义。
2. 结果
2.1 CCl4急性造模后各组小鼠肝脏病理观察
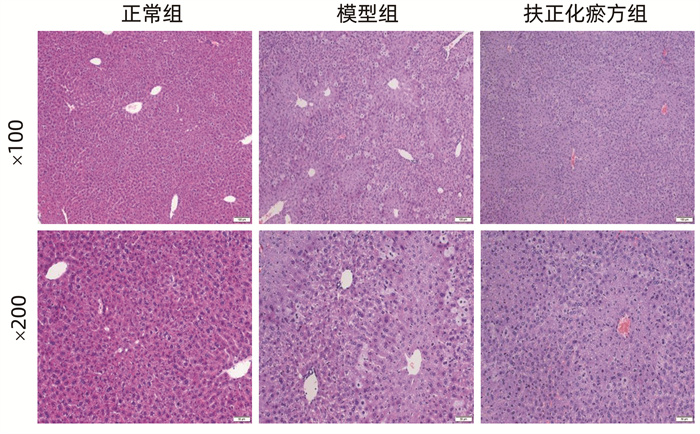
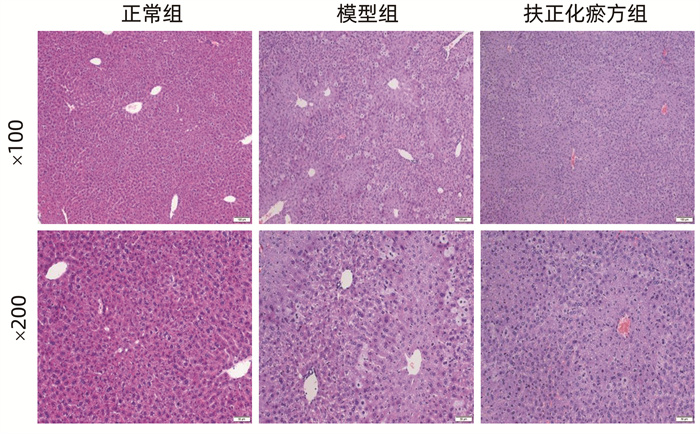
HE染色显示,正常组小鼠肝小叶结构完整,肝窦无充血、出血,肝细胞排列整齐、形成肝板,门静脉周围及汇管区无明显炎症细胞浸润和肝细胞变性坏死。模型组小鼠肝组织结构紊乱,肝细胞多胞浆疏松、水样变性,散在气球样变,见灶性坏死。扶正化瘀方组小鼠肝细胞变性、坏死程度较模型组有所减轻(图 1)。
2.2 CCl4急性造模后各组小鼠血清ALT和AST水平变化
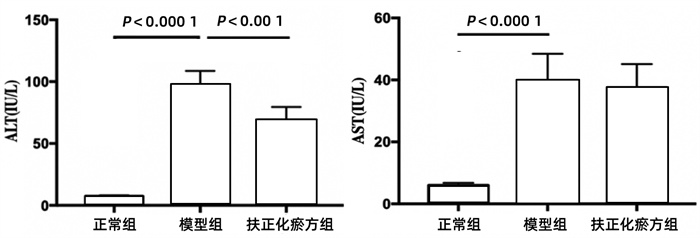
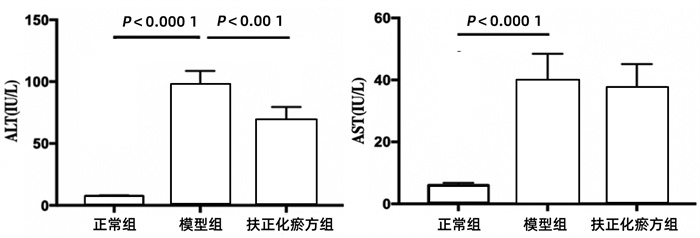
模型组的ALT和AST水平均显著高于正常组(P值均<0.000 1),经扶正化瘀方预防用药5 d的扶正化瘀方组ALT升高的幅度明显小于模型组(P<0.001)(图 2)。
2.3 各组肝脏相关细胞占总淋巴细胞比例
3组间总淋巴细胞、CD45、CD4-CD8+T、CD8+CD28-T比较差异均有统计学意义(P值均<0.01)(表 2)。
表 2 各组肝脏相关细胞占总淋巴细胞比例分组 动物数(只) 总淋巴细胞(个/mL) CD45(%) CD4-CD8+T(%) CD8+CD28-T(%) 正常组 6 8.62×106±0.425×106 40.20±1.31 3.96±0.152 3.73±0.251 模型组 6 2.53×107±0.157×107 58.80±1.90 1.37±0.152 1.27±0.153 扶正化瘀方组 6 1.69×107±0.447×107 29.60±1.43 1.63±0.115 1.67±0.152 F值 316.846 265.604 260.143 143.394 P值 <0.001 <0.001 <0.001 <0.001 2.4 JS 1与CCl4急性肝损伤小鼠肝脏CD8+ T淋巴细胞共培养24 h及48 h后α-SMA mRNA、Col.ⅠmRNA的表达变化
2.4.1 α-SMA mRNA
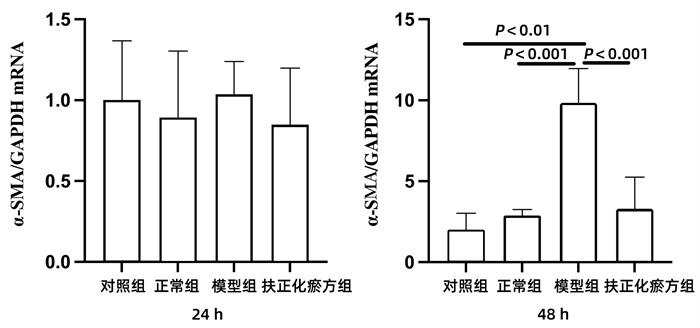
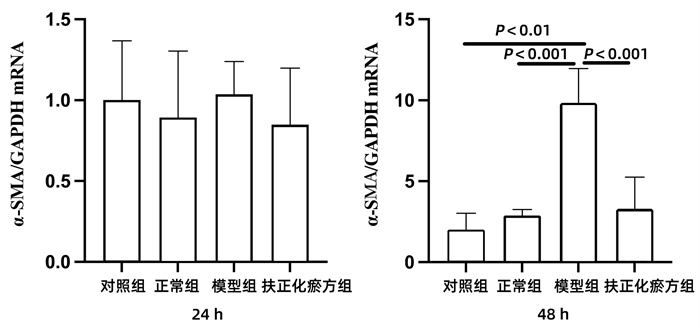
对照组(JS 1单培养)、正常组(JS 1与正常小鼠肝脏的CD8+ T淋巴细胞共培养)、模型组(JS 1与CCl4急性肝损伤小鼠肝脏的CD8+ T淋巴细胞共培养)、扶正化瘀方组(经扶正化瘀方治疗的CCl4急性肝损伤小鼠肝脏分离的CD8+ T淋巴细胞与JS 1共培养)在24 h时JS 1表达α-SMA mRNA相似,各组间差异均无统计学意义(P值均>0.05)。但是共培养到48 h时,与对照组和正常组相比,模型组α-SMA mRNA的表达均明显升高(P值均<0.01)。扶正化瘀方组的α-SMA mRNA表达升高则不明显,与模型组间的差异具有统计学意义(P<0.01)(图 3)。
2.4.2 Col.Ⅰ mRNA
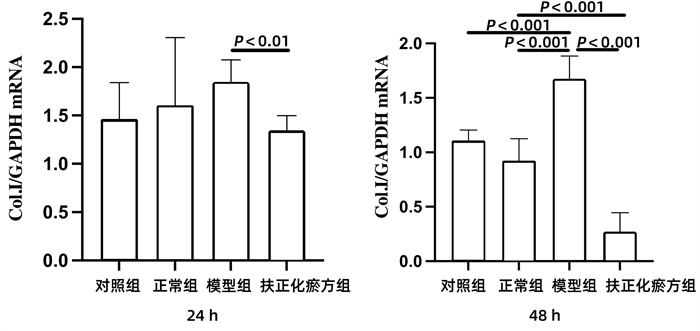
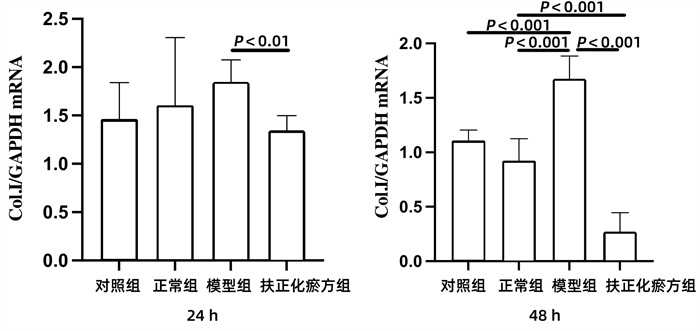
在共培养24 h时间点,与模型组相比,扶正化瘀方组的JS 1表达Col.ⅠmRNA明显下降(P<0.01)。在共培养到48 h时,与对照组和正常组相比,模型组JS 1表达Col.ⅠmRNA上升明显(P值均<0.001);扶正化瘀方组JS 1表达Col.ⅠmRNA明显低于模型组和正常组(P值均<0.001)(图 4)。
3. 讨论
肝纤维化的发生机制相当复杂,其中HSC活化和凋亡受到免疫系统的调控,而细胞介导的免疫反应在造成肝损害方面尤为重要。肝脏富含先天免疫细胞,包括巨噬细胞、树突状细胞、中性粒细胞、炎症性单核细胞、NK细胞和自然杀伤T淋巴细胞, 参与炎症反应[6-7]。肝内淋巴细胞、NK细胞、自然杀伤T淋巴细胞和黏膜相关的不变T淋巴细胞被确定为肝脏先天免疫系统中的重要淋巴细胞[8-10]。
活化的HSC以表达α-SMA为标志,同时产生大量的细胞外基质。细胞外基质的主要成分是胶原蛋白, 与肝纤维化相关的主要是Ⅰ、Ⅲ、Ⅳ型胶原。Ⅰ型和Ⅲ型胶原蛋白在纤维化肝中明显增多。Col.Ⅰ的沉积是肝纤维化发病机制的一个关键特征[11]。
扶正化瘀复方是以中医理论为指导,根据“虚损生积”,即“正虚血瘀”的病机特点,由丹参、发酵虫草菌粉、桃仁、松花粉、绞股蓝、五味子(制)6味中药配伍而成。具有“益精养肝,活血祛瘀”的作用。研究[12-14]发现,扶正化瘀方可以通过降低炎症因子的表达,直接或间接调节机体免疫功能,从而发挥抗肝纤维化作用,利于肝脏功能的恢复。有研究[15]使用流式细胞术对荷瘤昆明小鼠T淋巴细胞亚群及肿瘤细胞凋亡开展研究,发现经扶正化瘀方中中药成分桃仁的重要化学组成成分桃仁总蛋白治疗后,明显改善荷瘤小鼠CD4+/CD8+的细胞失衡状态, 恢复机体正常的免疫状态, 从而发挥抗肿瘤的作用。这些资料均提示,扶正化瘀方可能通过调节机体的免疫状态来抗肝纤维化。
本实验对扶正化瘀方进行研究发现:与对照组相比,在24 h及48 h时间点,正常组α-SMA、Col.Ⅰ的mRNA表达变化均不太明显,这与课题组以往以健康者外周血CD8+T淋巴细胞与LX-2共培养和LX-2单培养24 h后的研究结果趋势一致[16],说明正常CD8+T淋巴细胞并不刺激HSC的活化。
发生炎症反应的慢性肝炎患者由于肝脏内CD8+T淋巴细胞过度存在,不能清除抗原,致使炎症反应持续存在,促使CD8+T淋巴细胞活化,导致HSC的活化,最终导致纤维化的发生[17]。但是一项Meta分析[18]发现,CD8+T淋巴细胞或颗粒酶B产生的增加、CD4+T淋巴细胞的减少对滤泡性肿瘤预后十分有利,且CD8+T淋巴细胞可能是滤泡性肿瘤良好预后的标志性指标。Klametha等[19]对140例病毒感染和外周T淋巴细胞瘤患者外周血行流式细胞术后发现,其CD3+、CD8-、CD7-T淋巴细胞会反应性增加。而在HCV患者经过直接抗病毒药物治疗后,CD8+T淋巴细胞衰老及功能指标有所改善,利于肝纤维化治疗[20]。是否因为不同疾病中CD8+T淋巴细胞表型发生改变,故而其持续增加在不同疾病中所发生的作用也不尽相同?那么CD8+T淋巴细胞在小鼠CCl4急性肝损伤模型中是产生何种作用值得思考。
本实验对C57小鼠预防给药扶正化瘀方5 d后再造模,获得成功。病理观察提示:经扶正化瘀方预先用药后,扶正化瘀方组小鼠肝细胞变性、坏死程度较模型组均有所减轻;对小鼠血清检测发现:扶正化瘀方组小鼠AST水平变化与模型组相似,但是ALT升高的幅度明显小于模型组,T淋巴细胞比例降低。以上结果提示,扶正化瘀方用药5 d,已显示保护肝细胞的药效。
将CD8+T淋巴细胞与JS 1共培养24 h,模型组的CD8+T淋巴细胞尚未显示出对JS 1的明显影响,但共培养到48 h,其促进JS 1活化的作用十分明显,该组JS 1表达α-SMA mRNA和Col.Ⅰ mRNA远高于对照组和正常组。说明扶正化瘀方预先给药5 d能显著减轻CCl4所致的小鼠急性肝损伤,扶正化瘀方能够转变肝脏的CD8+T淋巴细胞,使之由激活HSC促进肝纤维化,转变为抑制HSC活化,并且随着共培养时间的延长,抑制HSC活化的作用更加显著,进而减缓纤维化进程,甚至有可能逆转纤维化。这一结果与课题组以往研究[21]中,扶正化瘀方有效组患者外周血CD8+T淋巴细胞能显著减少共培养的LX-2中α-SMA mRNA的表达(P<0.05),而扶正化瘀方无效组患者外周血CD8+T淋巴细胞这种作用不明显的结果相一致。扶正化瘀方经治小鼠肝脏的CD8+T淋巴细胞与JS 1共培养48 h,抑制了JS 1的活化,可能是CD8+T淋巴细胞与共培养的JS 1直接接触的结果。此结果与临床扶正化瘀方治疗有效患者外周血CD8+T淋巴细胞及其亚群通过调节细胞因子的分泌,抑制共培养的HSC活化的观察结果不一致[16]。
以上研究结果均提示扶正化瘀方药物血清可能通过诱导小鼠肝脏CD8+ T淋巴细胞抑制共培养的JS 1活化,这种抑制作用可能主要不是通过细胞因子调控的,但尚不能明确区分药物对HSC的直接或间接的调控作用。
囿于现阶段分选技术的限制,如采用流式技术分选耗时长、对细胞损伤较大,影响活率和得率;目前市场上无快捷分离的CD28磁珠试剂盒可在小鼠应用,故动物实验暂时无法进一步研究CD8+T淋巴细胞亚群对HSC的调节作用,期待以后能在人肝脏中开展后续研究。
-
表 1 qPCR引物列表
Cat.No. TM值 名称 上游引物 下游引物 Tnt0338 85.1 GAPDH 5′AAA TGG TGA AGG TCG GTG TG 3′ 5′AGG TCA ATG AAG GGG TCG TT 3′ Tnt1328 81.1 α-SMA 5′CAC ACA CCT TTT AGA ACC CA 3′ 5′GTT TTC CCC CTT CCT CTT ACT 3′ Tnt1680a 85.9 Col.Ⅰ 5′TGA CTG GAA GAG CGG AGA GTA 3′ 5′GAC GGC TGA GTA GGG AAC AC 3′ 表 2 各组肝脏相关细胞占总淋巴细胞比例
分组 动物数(只) 总淋巴细胞(个/mL) CD45(%) CD4-CD8+T(%) CD8+CD28-T(%) 正常组 6 8.62×106±0.425×106 40.20±1.31 3.96±0.152 3.73±0.251 模型组 6 2.53×107±0.157×107 58.80±1.90 1.37±0.152 1.27±0.153 扶正化瘀方组 6 1.69×107±0.447×107 29.60±1.43 1.63±0.115 1.67±0.152 F值 316.846 265.604 260.143 143.394 P值 <0.001 <0.001 <0.001 <0.001 -
[1] DING BS, CAO Z, LIS R, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis[J]. Nature, 2014, 505(7481): 97-102. DOI: 10.1038/nature12681. [2] CAMPANA L, IREDALE JP. Regression of liver fibrosis[J]. Semin Liver Dis, 2017, 37(1): 1-10. DOI: 10.1055/s-0036-1597816. [3] IRSHAD M, GUPTA P, IRSHAD K. Immunopathogenesis of liver injury during hepatitis C virus infection[J]. Viral Immunol, 2019, 32(3): 112-120. DOI: 10.1089/vim.2018.0124. [4] XING YM. Current status and prevention of chronic hepatitis B infection[J]. J World Latest Med Inf, 2017, 69: 174-175. DOI: 10.19613/j.cnki.1671-3141.2017.69.130.邢亚明. 慢性乙型肝炎感染现状及预防[J]. 世界最新医学信息文摘, 2017, 69: 174-175. DOI: 10.19613/j.cnki.1671-3141.2017.69.130. [5] LIU CH, LIU P, LIU C, et al. Discussion on serum pharmacological methods of effective Chinese herbal compound prescriptions for anti-liver fibrosis[J]. Chin J Exp Med Formul, 1998, 2: 16-19. https://www.cnki.com.cn/Article/CJFDTOTAL-ZSFX802.006.htm刘成海, 刘平, 刘成, 等. 抗肝纤维化有效中药复方血清药理学方法探讨[J]. 中国实验方剂学杂志, 1998, 2: 16-19. https://www.cnki.com.cn/Article/CJFDTOTAL-ZSFX802.006.htm [6] KOYAMA Y, BRENNER DA. Liver inflammation and fibrosis[J]. J Clin Invest, 2017, 127(1): 55-64. DOI: 10.1172/JCI88881. [7] NOTAS G, KISSELEVA T, BRENNER D. NK and NKT cells in liver injury and fibrosis[J]. Clin Immunol, 2009, 130(1): 16-26. DOI: 10.1016/j.clim.2008.08.008. [8] SCHUSTER S, CABRERA D, ARRESE M, et al. Triggering and resolution of inflammation in NASH[J]. Nat Rev Gastroenterol Hepatol, 2018, 15(6): 349-364. DOI: 10.1038/s41575-018-0009-6. [9] ARRESE M, CABRERA D, KALERGIS AM, et al. Innate immunity and inflammation in NAFLD/NASH[J]. Dig Dis Sci, 2016, 61(5): 1294-1303. DOI: 10.1007/s10620-016-4049-x. [10] JENNE CN, KUBES P. Immune surveillance by the liver[J]. Nat Immunol, 2013, 14(10): 996-1006. DOI: 10.1038/ni.2691. [11] CHEN Y, OU Y, DONG J, et al. Osteopontin promotes collagen I synthesis in hepatic stellate cells by miRNA-129-5p inhibition[J]. Exp Cell Res, 2018, 362(2): 343-348. DOI: 10.1016/j.yexcr.2017.11.035. [12] WU P, TAN XY. The effect of Fuzheng Huayu capsule on immine regulation and anti-fibrosis in hepatic C virus infected patients with decompensated cirrhosis[J]. Chin J Integr Trad West Med Dig, 2016, 24(9): 658-660. DOI: 10.3969/j.issn.1671-038X.2016.09.02.吴平, 谭小燕. 扶正化瘀胶囊对丙型肝炎失代偿期肝硬化患者免疫调节以及抗纤维化作用研究[J]. 中国中西医结合消化杂志, 2016, 24(9): 658-660. DOI: 10.3969/j.issn.1671-038X.2016.09.02. [13] CHEN H, YANG BW, YUAN M, et al. Prevention and therapeutic effects of Fuzheng Huayu capsule on liver fibrosis and expression of connective tissue growth factor in rats[J]. J Sichuan Univ (Med Sci Edi), 2016, 47(2): 197-202. https://www.cnki.com.cn/Article/CJFDTOTAL-HXYK201602012.htm陈晗, 杨碧伟, 袁满, 等. 扶正化瘀胶囊对肝纤维化大鼠的防治作用及对结缔组织生长因子表达的影响[J]. 四川大学学报(医学版), 2016, 47(2): 197-202. https://www.cnki.com.cn/Article/CJFDTOTAL-HXYK201602012.htm [14] WANG J. The effect of Fuzheng Huayu capsule assisting western medicine on intestinal mucosal barrier function, immune function and liver function in patients with hepatitis B liver cirrhosis[J]. Mod J Integr Tradit Chin West Med, 2017, 26(27): 3051-3053. DOI: 10.3969/j.issn.1008-8849.2017.27.031.王军. 扶正化瘀胶囊辅助西医对乙肝肝硬化患者肠黏膜屏障功能、免疫功能及肝功能状态的影响[J]. 现代中西医结合杂志, 2017, 26(27): 3051-3053. DOI: 10.3969/j.issn.1008-8849.2017.27.031. [15] XU HY, YUN CX, WANG YX. The effects of PSP on the subset of T cell and apoptosis of the tumo-bearing mice[J]. J Qiqihar Med Coll, 2004, 25(5): 485-487. DOI: 10.3969/j.issn.1002-1256.2004.05.003.许惠玉, 运晨霞, 王雅贤. 桃仁总蛋白对荷瘤鼠T淋巴细胞亚群及细胞凋亡的影响[J]. 齐齐哈尔医学院学报, 2004, 25(5): 485-487. DOI: 10.3969/j.issn.1002-1256.2004.05.003. [16] MA YL. Study on the immune regulation mechanism of Fuzheng Huayu recipe against liver fibrosis based on the changes of CD8+ T lymphocyte subsets[D]. ShangHai: ShangHai University of Traditional Chinese Medicine, 2017.马亚丽. 基于CD8+T淋巴细胞亚群的变化探讨扶正化瘀方抗肝纤维化的免疫调节机制[D]. 上海: 上海中医药大学, 2017. [17] SPAHN J, PIERCE RH, CRISPE IN. Ineffective CD8+ T-cell immunity to adeno-associated virus can result in prolonged liver injury and fibrogenesis[J]. Am J Pathol, 2011, 179(5): 2370-2381. DOI: 10.1016/j.ajpath.2011.08.004. [18] XIE M, JIANG Q, ZHAO S, et al. Prognostic value of tissue-infiltrating immune cells in tumor microenvironment of follicular lymphoma: A Meta-analysis[J]. Int Immunopharmacol, 2020, 85: 106684. DOI: 10.1016/j.intimp.2020.106684. [19] KLAMETHA, NEUBAUER A, KELLER C, et al. Aberrant CD3-positive, CD8-low, CD7-negative lymphocytes may appear during viral infections and mimic peripheral T-cell lymphoma[J]. Diagnostics, 2020, 10(4): 204. DOI: 10.3390/diagnostics10040204. [20] ZHANG PX, BIAN PY, YE CT, et al. Changes in senescence-and function-related parameters of CD8+T cells after direct-acting antiviral treatment in patients with hepatitis C virus infection[J]. J Clin Hepatol, 2020, 36(7): 1496-1501. DOI: 10.3969/j.issn.1001-5256.2020.07.011.张沛欣, 边培育, 叶传涛, 等. HCV感染者直接抗病毒药物治疗前后CD8+T淋巴细胞衰老和功能相关指标的变化[J]. 临床肝胆病杂志, 2020, 36(7): 1496-1501. DOI: 10.3969/j.issn.1001-5256.2020.07.011. 期刊类型引用(5)
1. 李璐,孙建光,王磊. “形不足者,温之以气;精不足者,补之以味”在肝硬化治疗中的应用. 中西医结合肝病杂志. 2024(05): 421-423 .  百度学术
百度学术2. 王鹏,司东晓,余令兵. 扶正化瘀胶囊联合异甘草酸镁治疗HBV感染失代偿期肝硬化患者的疗效分析. 罕少疾病杂志. 2024(09): 85-87 .  百度学术
百度学术3. 杨逸鑫,彭芳. 基于代谢组学的中医药防治肝纤维化的研究进展. 中南药学. 2023(01): 155-160 .  百度学术
百度学术4. 杨耀. 中医中药抗肝纤维化作用机制及临床应用研究进展. 现代养生. 2023(05): 321-323 .  百度学术
百度学术5. 高志远,何新颖,许迪,祁月英,徐湘江. 益肝化湿饮对乙肝肝纤维化患者T淋巴细胞亚群的影响. 中国中西医结合消化杂志. 2023(07): 562-566 .  百度学术
百度学术其他类型引用(0)
-




 PDF下载 ( 2677 KB)
PDF下载 ( 2677 KB)

 下载:
下载:




 下载:
下载:
 百度学术
百度学术