PLT水平及PLT参数对慢加急性肝衰竭患者预后的影响
DOI: 10.3969/j.issn.1001-5256.2022.02.023
Effect of platelet level and platelet parameters on the prognosis of patients with acute-on-chronic liver failure
-
摘要:
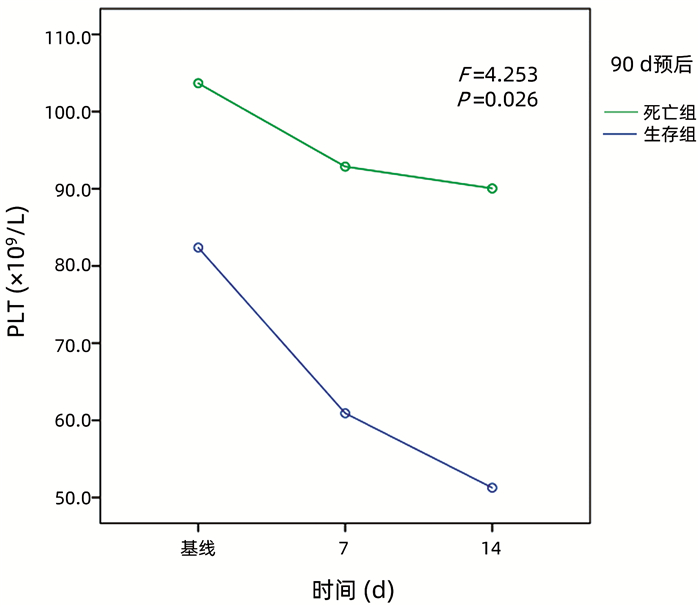
目的 探讨PLT及PLT参数在不同分型、不同病因的慢加急性肝衰竭(ACLF)患者中的差异,评估PLT和PLT的动态变化对ACLF患者预后的影响。 方法 收集2014年1月—2018年12月在天津市第三中心医院就诊的364例ACLF患者的临床资料和肝功能、PLT及PLT参数,比较不同分型及病因的ACLF患者PLT水平与PLT参数(PLT分布宽度、平均PLT体积),分析其对于ACLF患者90 d死亡率的影响,分析基线、入院后7 d、14 d PLT动态变化与患者预后的关系。计数资料组间比较采用χ2检验,计量资料组间比较采用Kruskal-Wallis H检验或Mann-Whitney U检验,生存分析用Kaplan-Meier法,预后相关参数采用单因素及多因素Cox回归分析,PLT动态变化用重复测量方差分析。根据PLT水平及总生存情况绘制ROC曲线。 结果 C型ACLF患者PLT水平在明显低于A型及B型ACLF患者(P值均<0.001);相比于乙型肝炎组ACLF患者,自身免疫性肝病的ACLF患者平均PLT体积降低(P=0.035);根据ROC曲线得出临界值,PLT<60.5×109/L的患者死亡率大于PLT≥60.5×109/L组(P=0.006);PLT水平是ACLF患者90 d死亡的独立保护性因素(HR=0.995, 95%CI: 0.990~0.999,P=0.026),PLT水平越低病死率越高;C型患者死亡率明显高于A型患者(P<0.05),死亡组PLT水平减少更明显(P<0.05);ACLF患者90 d死亡组PLT下降幅度明显高于生存组(P=0.032)。 结论 不同分型ACLF患者PLT水平存在差异,PLT水平是影响ACLF患者90 d预后的重要指标,PLT动态下降幅度大的ACLF患者90 d死亡率高。 Abstract:Objective To investigate the differences in platelet and platelet parameters between patients with different types and etiologies of acute-on-chronic liver failure (ACLF) and the influence of platelet and its dynamic change on the prognosis of ACLF patients. Methods Clinical data, liver function parameters, platelet, and platelet parameters were collected from 364 patients with ACLF who attended Tianjin Third Central Hospital from January 2014 to December 2018. Platelet level and platelet parameters (platelet distribution width and mean platelet volume) were compared between the patients with different types and etiologies of ACLF, and their influence on the 90-day mortality rate of ACLF patients was analyzed, as well as the association of the dynamic change of platelet at baseline and on days 7 and 14 after admission with the prognosis of patients. The chi-square test was used for comparison of categorical data between groups; the Kruskal-Wallis H test or Mann-Whitney U test was used for comparison of continuous data between groups; the Kaplan-Meier method was used for survival analysis; the univariate and multivariate Cox regression analyses were used to analyze the parameters associated with prognosis; the repeated measures analysis of variance was used to analyze the dynamic change of platelet; receiver operating characteristic (ROC) curve was plotted based on platelet level and overall survival. Results The patients with type C ACLF had a significantly lower platelet level than those with type A/B ACLF (all P < 0.001). Compared with the ACLF patients with hepatitis B, the ACLF patients with autoimmune liver diseases had a significant reduction in mean platelet volume (P=0.035). Based on the cut-off value obtained by the ROC curve analysis, the patients with a platelet level of < 60.5×109/L had a significantly higher mortality rate than those with a platelet level of ≥60.5×109/L (P=0.006). Platelet level was an independent protective factor against 90-day death in ACLF patients (hazard ratio=0.995, 95% confidence interval: 0.990-0.999, P=0.026), and the mortality rate increased with the reduction in platelet level. The patients with type C ACLF had a significantly higher mortality rate than those with type A ACLF (P < 0.05), and the death group tended to have a significantly greater reduction in platelet level (P < 0.05). Compared with the survival group, the 90-day death group had a significantly greater reduction in platelet (P=0.032). Conclusion There is a difference in platelet level between ACLF patients with different types. Platelet level is an important indicator for the 90-day prognosis of ACLF patients, and patients with a greater dynamic reduction in platelet tend to have a higher 90-day mortality rate. -
Key words:
- Acute-On-Chronic Liver Failure /
- Thrombocytopenia /
- Prognosis
-
表 1 ACLF患者的基线数据
项目 数值 年龄(岁) 54.0(47.0~61.0) 男[例(%)] 255(70.1) 分型[例(%)] A型 72(19.8) B型 173(47.5) C型 119(32.7) 病因[例(%)] 乙型肝炎 172(47.3) 丙型肝炎 12(3.3) 酒精性肝炎 102(28.0) 自身免疫性肝病 42(11.5) 代谢相关脂肪性肝病 2(0.5) 药物性肝损伤 5(1.4) 病因不详 28(7.7) 实验室指标 ALT(U/L) 86(6~2958) Alb(g/L) 27.8(14.0~44.2) TBil(μmol/L) 206.7(26.7~682.7) Na(mmol/L) 134.9(112.9~151.0) Cr(μmol/L) 66(24~580) WBC(×109/L) 6.70(1.63~38.60) PLT(×109/L) 85(14~613) INR 2.06(0.89~6.96) 合并症[例(%)] 感染 233(64.0) 消化道出血 53(14.6) 肾功能不全 59(16.2) MELD评分 20.1(5.4~42.7) 累计死亡率[例(%)] 28 d 54(14.8) 90 d 96(26.4) 表 2 不同分型ACLF患者实验室指标及PLT参数水平
实验室指标 A型组(n=72) B型组(n=173) C型组(n=119) χ2值 P值 ALT(U/L) 449(7~2716) 88(9~2958)1) 40(6~2240)1)2) 72.900 <0.001 Alb(g/L) 31.2(18.7~44.2) 27.9(18.9~41.1)1) 26.3(14.0~38.8)1)2) 20.826 <0.001 TBil(μmol/L) 233.4(63.1~539.3) 230.0(30.1~673.4) 134.2(26.7~682.7)1)2) 29.689 <0.001 Na(mmol/L) 136.5(117.6~142.8) 135.1(114.0~147.1)1) 133.1(112.9~151.0)1)2) 22.094 <0.001 Cr(μmol/L) 60(27~214) 64(24~440) 81(30~280)1)2) 14.913 0.001 WBC(×109/L) 6.08(2.27~23.99) 6.69(1.74~36.72) 7.20(1.63~38.60) 1.083 0.582 PLT(×109/L) 123.5(20.0~388.0) 85.0(14.0~613.0)1) 62.0(14.0~236.0)1)2) 56.041 <0.001 PDW(fL) 55.2(17.9~76.7) 55.8(16.6~72.0) 57.5(17.6~73.1) 4.764 0.092 MPV(fL) 10.3(8.0~12.7) 10.3(7.4~17.8) 10.2(7.6~14.4) 0.657 0.720 注:PDW,PLT分布宽度;MPV,平均PLT体积。与A型组比较,1)P<0.05;与B型组比较,2)P<0.05。 表 3 不同病因ACLF患者实验室指标及PLT参数水平
实验室指标 乙型肝炎组
(n=172)酒精性肝炎组
(n=105)自身免疫性肝炎组
(n=42)χ2值 P值 ALT(U/L) 193(16~2916) 36(8~2958)1) 87(8~918)1)2) 59.953 <0.001 Alb(g/L) 28.7(17.3~41.1) 26.8(18.7~35.4)1) 27.6(14.0~36.3)1) 12.033 0.006 TBil(μmol/L) 189.0(38.2~539.2) 212.0(32.4~673.4) 170.6(30.1~682.7) 3.771 0.399 Na(mmol/L) 135.6(114.0~144.3) 133.5(112.9~147.1) 135.0(120.2~142.7) 6.325 0.092 Cr(μmol/L) 65(24~580) 67(35~440) 58.5(30~142)1)2) 13.823 0.004 WBC(×109/L) 6.12(1.74~27.60) 7.85(2.44~38.60) 5.00(1.63~10.79) 21.690 <0.001 PLT(×109/L) 83(19~247) 85(14~613) 78(14~237) 1.228 0.541 PDW(fL) 57.7(51.3~76.7) 55.8(19.4~71.1) 54.0(47.7~71.7) 2.358 0.308 MPV(fL) 10.4(7.4~14.7) 10.3(7.6~17.8) 9.9(8.3~14.4)1)2) 6.680 0.035 注:与乙型肝炎组比较,1)P<0.05;与酒精性肝炎组比较,2)P<0.05。 表 4 ACLF患者90 d死亡风险单因素及多因素分析
变量 单因素分析 多因素分析 HR 95%CI P值 HR 95%CI P值 MELD评分 1.071 1.041~1.102 <0.001 1.035 0.994~1.078 0.092 年龄 1.039 1.020~1.060 <0.001 1.033 1.013~1.053 0.001 性别 1.452 0.958~2.201 0.078 ALT 1.000 0.999~1.000 0.059 TBil 1.002 1.001~1.004 0.003 1.002 1.001~1.004 0.010 INR 1.223 0.979~1.527 0.076 Cr 1.005 1.003~1.007 <0.001 1.002 0.999~1.005 0.231 Na 0.960 0.927~0.994 0.022 0.995 0.955~1.036 0.798 WBC 1.016 0.979~1.053 0.404 PLT 0.996 0.992~0.999 0.023 0.995 0.990~0.999 0.026 PDW 0.992 0.972~1.011 0.393 MPV 1.038 0.897~1.201 0.615 表 5 不同病因、不同分型和PLT动态变化对生存的影响
项目 死亡组(n=68) 生存组(n=182) 统计值 P值 病因[例(%)] χ2=0.475 0.788 乙型肝炎 27(39.7) 77(42.3) 酒精性肝炎 21(30.9) 48(26.4) 自身免疫性肝炎组 9(13.2) 21(11.5) 分型[例(%)] χ2=6.124 0.047 A型 8(11.8) 44(24.2) B型 32(47.0) 86(47.2) C型 28(41.2)1) 52(28.6)1) PLT水平(×109/L) 基线 82(19~197) 106(14~613) Z=-1.979 0.048 第7天 61(10~178)2) 93(12~345)2) Z=-3.669 <0.001 第14天 51(10~136)2) 90(15~329)2) Z=-5.185 <0.001 14天与基线变化 -31(-133~82) -16(-457~176) Z=-2.875 0.004 注:与同组A型比较,1)P<0.05;与同组基线比较,2)P<0.05。 -
[1] GUSTOT T, FERNANDEZ J, GARCIA E, et al. Clinical course of acute-on-chronic liver failure syndrome and effects on prognosis[J]. Hepatology, 2015, 62(1): 243-252. DOI: 10.1002/hep.27849. [2] LI YL, YANG XL, LIU XJ, et al. Regulatory effects of immune cells on acute-on-chronic liver failure[J/CD]. Chin J Liver Dis (Electronic Version), 2020, 12(2): 42-46. DOI: 10.3969/j.issn.1674-7380.2020.02.008.李彦霖, 杨雪亮, 刘小静, 等. 免疫细胞在慢加急性肝衰竭中的调控作用[J/CD]. 中国肝脏病杂志(电子版), 2020, 12(2): 42-46. DOI: 10.3969/j.issn.1674-7380.2020.02.008. [3] ISHIKAWA T, ICHIDA T, MATSUDA Y, et al. Reduced expression of thrombopoietin is involved in thrombocytopenia in human and rat liver cirrhosis[J]. J Gastroenterol Hepatol, 1998, 13(9): 907-913. DOI: 10.1111/j.1440-1746.1998.tb00760.x. [4] ASTER RH. Pooling of platelets in the spleen: Role in the pathogenesis of "hypersplenic" thrombocytopenia[J]. J Clin Invest, 1966, 45(5): 645-657. DOI: 10.1172/JCI105380. [5] OLARIU M, OLARIU C, OLTEANU D. Thrombocytopenia in chronic hepatitis C[J]. J Gastrointestin Liver Dis, 2010, 19(4): 381-385. [6] LISMAN T, BONGERS TN, ADELMEIJER J, et al. Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity[J]. Hepatology, 2006, 44(1): 53-61. DOI: 10.1002/hep.21231. [7] Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35(1): 38-44. DOI: 10.3969/j.issn.1001-5256.2019.01.007.中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2018年版)[J]. 临床肝胆病杂志, 2019, 35(1): 38-44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. [8] Chinese Society of Hepatology, Chinese Medical Association. Chinese guidelines on the management of liver cirrhosis[J]. J Clin Hepatol, 2019, 35(11): 2408-2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006.中华医学会肝病学分会. 肝硬化诊治指南[J]. 临床肝胆病杂志, 2019, 35(11): 2408-2425. DOI: 10.3969/j.issn.1001-5256.2019.11.006. [9] MALINCHOC M, KAMATH PS, GORDON FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts[J]. Hepatology, 2000, 31(4): 864-871. DOI: 10.1053/he.2000.5852. [10] JALAN R, YURDAYDIN C, BAJAJ JS, et al. Toward an improved definition of acute-on-chronic liver failure[J]. Gastroenterology, 2014, 147(1): 4-10. DOI: 10.1053/j.gastro.2014.05.005. [11] KHODADI E. Platelet function in cardiovascular disease: Activation of molecules and activation by molecules[J]. Cardiovasc Toxicol, 2020, 20(1): 1-10. DOI: 10.1007/s12012-019-09555-4. [12] SHIDO K, CHAVEZ D, CAO Z, et al. Platelets prime hematopoietic and vascular niche to drive angiocrine-mediated liver regeneration[J]. Signal Transduct Target Ther, 2017, 2: 16044. DOI: 10.1038/sigtrans.2016.44. [13] TAKAHASHI K, NAGAI S, COLLINS KM, et al. Factors associated with low graft regeneration in the early phase after living donor liver transplantation[J]. Clin Transplant, 2019, 33(10): e13690. DOI: 10.1111/ctr.13690. [14] GIANNINI EG, GRECO A, MARENCO S, et al. Incidence of bleeding following invasive procedures in patients with thrombocytopenia and advanced liver disease[J]. Clin Gastroenterol Hepatol, 2010, 8(10): 899-902; quiz e109. DOI: 10.1016/j.cgh.2010.06.018. [15] ABDEL-RAZIK A, ELDARS W, RIZK E. Platelet indices and inflammatory markers as diagnostic predictors for ascitic fluid infection[J]. Eur J Gastroenterol Hepatol, 2014, 26(12): 1342-1347. DOI: 10.1097/MEG.0000000000000202. [16] EKIZ F, YÜKSEL O, KOÇAK E, et al. Mean platelet volume as a fibrosis marker in patients with chronic hepatitis B[J]. J Clin Lab Anal, 2011, 25(3): 162-165. DOI: 10.1002/jcla.20450. [17] HAN L, HAN T, NIE C, et al. Elevated mean platelet volume is associated with poor short-term outcomes in hepatitis B virus-related acute-on-chronic liver failure patients[J]. Clin Res Hepatol Gastroenterol, 2015, 39(3): 331-339. DOI: 10.1016/j.clinre.2014.10.006. [18] XU Y, HUANG XP, CHEN L, et al. Value of mean platelet volume in evaluating the prognosis of hepatitis B virus associated acute on chronic liver failure[J]. J Clin Hepatol, 2020, 36(10): 2199-2202. DOI: 10.3969/j.issn.1001-5256.2020.10.008.徐英, 黄小平, 陈丽, 等. 平均PLT体积对HBV相关慢加急性肝衰竭预后的评估价值[J]. 临床肝胆病杂志, 2020, 36(10): 2199-2202. DOI: 10.3969/j.issn.1001-5256.2020.10.008. [19] ABDEL-RAZIK A, MOUSA N, ZAKARIA S, et al. New predictive factors of poor response to therapy in autoimmune hepatitis: Role of mean platelet volume[J]. Eur J Gastroenterol Hepatol, 2017, 29(12): 1373-1379. DOI: 10.1097/MEG.0000000000000982. [20] CHAUHAN A, ADAMS DH, WATSON SP, et al. Platelets: No longer bystanders in liver disease[J]. Hepatology, 2016, 64(5): 1774-1784. DOI: 10.1002/hep.28526. [21] MILOVANOVIC ALEMPIJEVIC T, STOJKOVIC LALOSEVIC M, DUMIC I, et al. Diagnostic accuracy of platelet count and platelet indices in noninvasive assessment of fibrosis in nonalcoholic fatty liver disease patients[J]. Can J Gastroenterol Hepatol, 2017, 2017: 6070135. DOI: 10.1155/2017/6070135. [22] DELGADO-GARCÍA G, GALARZA-DELGADO DÁ, COLUNGA-PEDRAZA I, et al. Mean platelet volume is decreased in adults with active lupus disease[J]. Rev Bras Reumatol Engl Ed, 2016, 56(6): 504-508. DOI: 10.1016/j.rbre.2016.03.003. [23] KISACIK B, TUFAN A, KALYONCU U, et al. Mean platelet volume (MPV) as an inflammatory marker in ankylosing spondylitis and rheumatoid arthritis[J]. Joint Bone Spine, 2008, 75(3): 291-294. DOI: 10.1016/j.jbspin.2007.06.016. [24] PENG YF, HUANG YX, WEI YS. Altered mean platelet volume in patients with polymyositis and its association with disease severity[J]. Braz J Med Biol Res, 2016, 49(6): e5168. DOI: 10.1590/1414-431X20165168. [25] YANG MR, ZHOU ZP. Update of clinical characteristics and treatment of secondary ITP from autoimmune diseases[J]. Chin J Immunol, 2021, 37(3): 361-366. DOI: 10.3969/j.issn.1000-484X.2021.03.019.杨慕然, 周泽平. 免疫性疾病继发性ITP临床特点及治疗研究进展[J]. 中国免疫学杂志, 2021, 37(3): 361-366. DOI: 10.3969/j.issn.1000-484X.2021.03.019. [26] SCHMOELLER D, PICARELLI MM, PAZ MUNHOZ T, et al. Mean platelet volume and immature platelet fraction in autoimmune disorders[J]. Front Med (Lausanne), 2017, 4: 146. DOI: 10.3389/fmed.2017.00146. -




 PDF下载 ( 2578 KB)
PDF下载 ( 2578 KB)

 下载:
下载: