基于文本知识库的肝损伤药物不良反应大数据智能识别研究
DOI: 10.3969/j.issn.1001-5256.2022.02.024
利益冲突声明: 本研究不存在研究者、伦理委员会成员、受试者监护人以及与公开研究成果有关的利益冲突,特此声明。
作者贡献声明: 葛斐林负责分析数据,撰写文章;牛明、赵旭、柏兆方负责整理数据;肖小河负责论文的修改;郭玉明、王伽伯负责拟定论文思路,指导撰写文章并最后定稿。
Intelligent identification of the big data of liver injury-related adverse drug reactions based on text database
-
摘要:
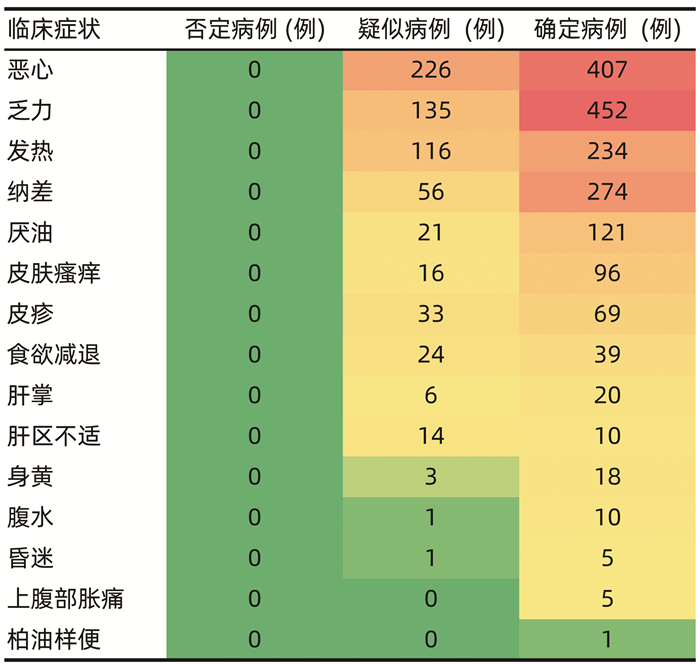
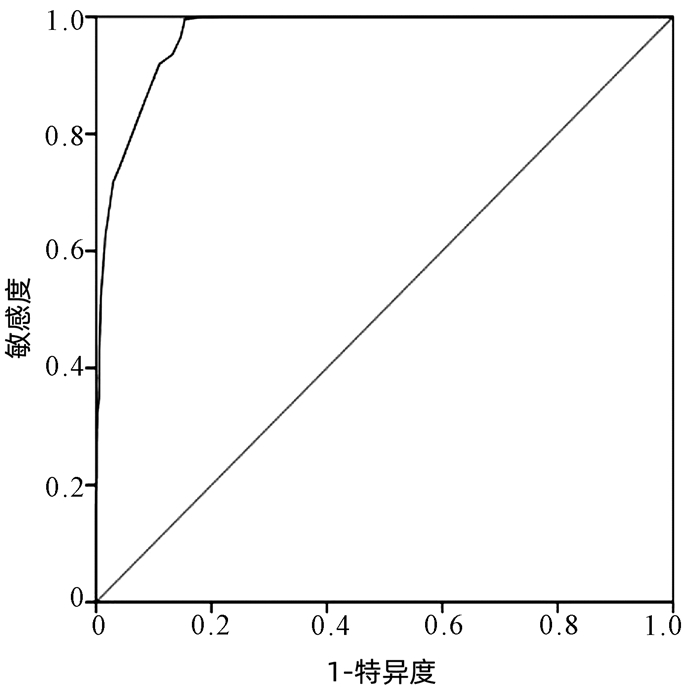
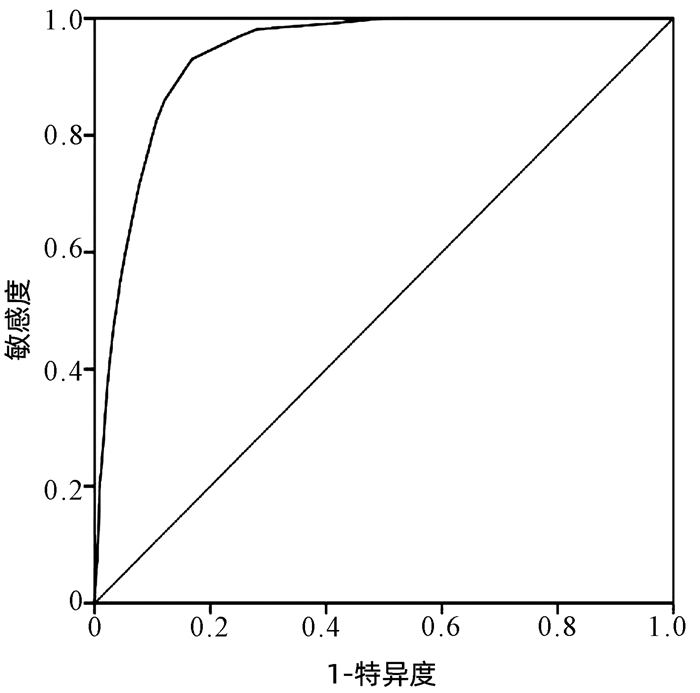
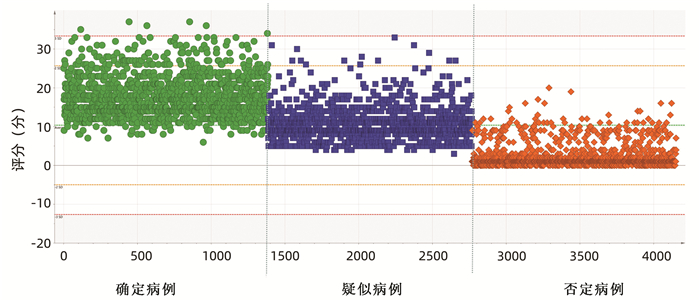
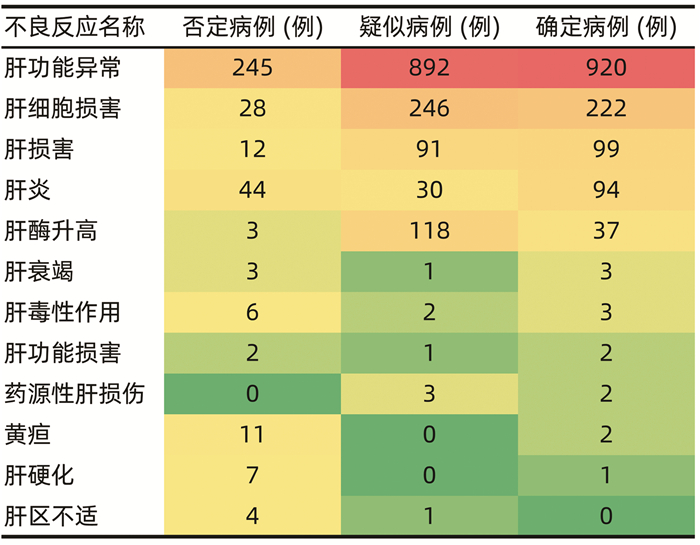
目的 本研究基于药物不良反应(ADR)文本知识库的探索性构建,尝试建立肝损伤相关ADR的大数据智能识别方法。 方法 以“药物性肝损伤”“药源性肝损伤”“肝功能异常”等为关键词,检索时间为2012年1月1日—2016年12月31日,检索并随机抽取药品不良反应监测系统数据库中5%(4152份)肝损伤相关ADR病例报告。结合医师临床再评价,分为“否定病例”“疑似病例”“确定病例”。在此基础上,进行关键要素的识别(不良反应名称、生化指标、临床症状),采用关键要素与临床再评价的相关性分析,以及ROC曲线确定评分阈值等构建肝损伤相关ADR智能识别方法,并采用交叉验证的方法评价该智能识别方法的效能。 结果 肝损伤相关ADR评价识别公式为:总分(M)=症状分数+指标分数+不良反应名称分数,“否定病例”与“疑似病例”“确定病例”在M=5分区分度最好(AUC=0.97),敏感度为99.57%,特异度为84.61%;“确定病例”与“疑似病例”“否定病例”在M=12分区分度最好(AUC=0.938),敏感度为87.93%,特异度为85.98%。 结论 该方法将为肝损伤相关ADR大数据智能识别评价提供参考和依据,有望有效减轻人工处理肝损伤相关ADR大数据的负担,为肝损伤相关ADR的早期风险信号识别及预警提供有效工具和方法学示范。 -
关键词:
- 肝疾病 /
- 药物相关性副作用和不良反应 /
- 知识库 /
- 人工智能
Abstract:Objective To establish the intelligent identification method for the big data of liver injury-related adverse drug reaction (ADR) based on the construction of text database. Methods With the keywords including "drug-induced liver injury" and "abnormal liver function" and a search time of January 1, 2012 to December 31, 2016, 5% (4152 cases) of the case reports of liver injury-related ADR were retrieved and extracted from the China Adverse Drug Reaction Monitoring System, and then based on clinical reevaluation by physicians, these cases were classified into "negative cases", "suspected cases", and "confirmed cases". On this basis, key elements (including ADR name, biochemical parameter, and clinical symptoms) were identified. An intelligent identification method for liver injury-related ADR was established based on the correlation analysis between key elements and clinical reevaluation and the receiver operating characteristic (ROC) curve for determining cut-off values, and the method of cross validation was used to evaluate the performance of this intelligent identification method. Results The formula for the evaluation and identification of liver injury-related ADR was as follows: total score (M)=symptom score+index score+ADR name score. This formula showed the best discriminatory ability to distinguish "negative case" from "suspected case" or "confirmed case" at M=5 (area under the ROC curve [AUC]=0.97), with a sensitivity of 99.57% and a specificity of 84.61%, and it showed the best discriminatory ability to distinguish "confirmed case" from "suspected case" or "negative case" at M=12 (AUC=0.938), with a sensitivity of 87.93% and a specificity of 85.98%. Conclusion This method provides reference and basis for intelligent identification and evaluation of big data on liver injury-related ADR and is expected to effectively reduce the burden of manual processing of ADR big data and provide effective tools and methodological demonstration for early risk signal identification and warning of liver injury-related ADR. -
[1] The State Council. Outline of "Healthy China 2030" plan[EB/OL]. (2016-10-25) http://www.gov.cn/xinwen/2016-10/25/content_5124174.htm.国务院. "健康中国2030 "规划纲要[EB/OL]. (2016-10-25)http://www.gov.cn/xinwen/2016-10/25/content_5124174.htm. [2] The State Council. The 13th Five-Year Plan for National Drug Safety[EB/OL]. (2017-02-21) http://www.gov.cn/xinwen/2017-02/21/content_5169808.htm.国务院. "十三五"国家药品安全规划[EB/OL]. (2017-02-21) http://www.gov.cn/xinwen/2017-02/21/content_5169808.htm. [3] Drug-Induced Liver Disease Study Group, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the management of drug-induced liver injury[J]. J Clin Hepatol, 2015, 31(11): 1752-1769. DOI: 10.3969/j.Issn.1001-5256.2015.11.002.中华医学会肝病学分会药物性肝病学组. 药物性肝损伤诊治指南[J]. 临床肝胆病杂志, 2015, 31(11): 1752-1769. DOI: 10.3969/j.issn.1001-5256.2015.11.002. [4] GE FL, NIU M, HAN ZX, et al. Landscape of hepatobiliary adverse drug reactions related to preparations containing psoraleae fructus and its application in pharmacovigilance[J]. Chin J Integr Med, 2021, 27(11): 832-837. DOI: 10.1007/s11655-021-3442-2.[Online ahead of print] [5] GE FL, GUO YM, CAO JL, et al. Research progress on evaluation methods and risk factors for Chinese medicines-induced liver injury[J]. Mod Chin Med, 2019, 21(3): 284-290. DOI: 10.13313/j.issn.1673-4890.20180925002.葛斐林, 郭玉明, 曹俊岭, 等. 中药药源性肝损伤评价方法及风险因素研究进展[J]. 中国现代中药, 2019, 21(3): 284-290. DOI: 10.13313/j.issn.1673-4890.20180925002. [6] DU XX, SONG HB, REN JT, et al. Opportunities and challenges of post-marketing evaluation of raditional Chinese medicine[J]. Chin J Chin Mater Med, 2014, 39(18): 3427-3429. DOI: 10.4268/cjcmm20141803.杜晓曦, 宋海波, 任经天, 等. 中药上市后评价的机遇与挑战[J]. 中国中药杂志, 2014, 39(18): 3427-3429. DOI: 10.4268/cjcmm20141803. [7] MAO YM. Strengthening the scientific research and supervision of drug-induced liver injury based on big data[J]. J Clin Hepatol, 2018, 34(6): 1166 -1168. DOI: 10.3969/j.issn.1001-5256.2018.06.005.茅益民. 加强基于大数据的药物性肝损伤的科学研究和监管[J]. 临床肝胆病杂志, 2018, 34(6): 1166-1168. DOI: 10.3969/j.issn.1001-5256.2018.06.005. [8] State Drug Administration. Technical guidelines for clinical evaluation of liver injury induced by traditional Chinese medicine[J]. J Clin Hepatol, 2018, 34(7): 1403-1409. DOI: 10.3969/j.issn.1001-5256.2018.07.008.国家药品监督管理局. 中药药源性肝损伤临床评价技术指导原则[J]. 临床肝胆病杂志, 2018, 34(7): 1403-1409. DOI: 10.3969/j.issn.1001-5256.2018.07.008. [9] World Health Organization (WHO), Uppsala Monitoring Centre. The use of the WHO-UMC system for standardised case causality assessment[EB/OL]. http://www.who-umc.org/Graphics/26649.pdf 2018. [10] PAN XC. Drug risk assessment based on cluster analysis[D]. Nanjing: Nanjing University of Posts and Telecommunications, 2020.潘轩超. 基于聚类分析的药物风险评估[D]. 南京: 南京邮电大学, 2020. [11] ZHU Q, DING J, REN WX, et al. Study on risk assessment model of in vitro diagnostic reagent adverse events based on BP neural network[J]. Chin J Med Instrument, 2019, 43(2): 136-139. DOI: 10.3969/j.issn.1671-7104.2019.02.017.朱清, 丁静, 任文霞, 等. 基于BP神经网络的体外诊断试剂不良事件风险评估模型研究[J]. 中国医疗器械杂志, 2019, 43(2): 136-139. DOI: 10.3969/j.issn.1671-7104.2019.02.017. [12] HARPAZ R, DUMOCHEL W, SHAH NH. Big data and adverse drug reaction detection[J]. Clin Pharmacol Ther, 2016, 99(3): 268-270. DOI: 10.1002/cpt.302. [13] MA HL, XU CY, YANG XM. Application status of decision tree in traditional Chinese medicine[J]. World Chin Med, 2021, 16(17): 2648-2651, 2656. DOI: 10.3969/j.issn.1673-7202.2021.17.025.马红丽, 徐长英, 杨新鸣. 决策树模型在中医药领域的应用现状[J]. 世界中医药, 2021, 16(17): 2648-2651, 2656. DOI: 10.3969/j.issn.1673-7202.2021.17.025. [14] GE FL, NIU M, HAN ZX, et al. Analysis of epidemiological characteristics of drug induced liver injury associated with baixianpi preparations[J]. Chin J Chin Mater Med, 2019, 44(5): 1048-1052. DOI: 10.19540/j.cnki.cjcmm.20181217.001.葛斐林, 牛明, 韩紫欣, 等. 白鲜皮制剂相关肝损伤的药物流行病学特征分析[J]. 中国中药杂志, 2019, 44(5): 1048-1052. DOI: 10.19540/j.cnki.cjcmm.20181217.001. -



 PDF下载 ( 3705 KB)
PDF下载 ( 3705 KB)

 下载:
下载: