线粒体靶向抗氧化剂SS-31对脓毒症小鼠模型急性肝损伤的影响
DOI: 10.3969/j.issn.1001-5256.2022.02.025
利益冲突声明:本研究不存在研究者、伦理委员会成员、受试者监护人以及与公开研究成果有关的利益冲突,特此声明。
作者贡献声明:满明银负责课题设计,资料分析,撰写论文;李娜娜参与收集数据,修改论文;卜月负责实验及修改论文;于凯江负责拟定写作思路,指导撰写文章并最后定稿。
Influence of mitochondria-targeted antioxidant SS-31 on acute liver injury in a mouse model of sepsis
-
摘要:
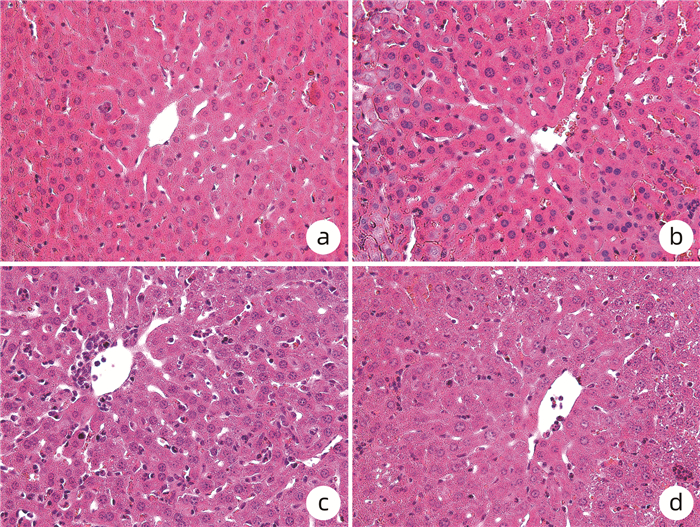
目的 探究线粒体靶向抗氧化剂SS-31对脓毒症小鼠模型急性肝损伤的作用。 方法 将24只C57BL/6J成年雄性小鼠随机分为control组、control+SS-31组、脂多糖(LPS)组(脓毒症组)、LPS+SS-31组,每组6只。小鼠腹腔注射LPS(10 mg/kg)制备脓毒症急性肝损伤模型或对照组腹腔注射等体积PBS液,继之腹腔注射SS-31(10 mg/kg)进行治疗或对照组腹腔注射等体积生理盐水,12 h后ELISA法检测ALT、AST、活性氧(ROS)、超氧化物歧化酶(SOD)、TNFα、IL-1β、IL-6水平,HE染色观察肝脏组织病理学。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 与LPS组相比,LPS+SS-31组小鼠血清中ALT[(140.05±12.22) U/L vs (102.64±8.75)U/L]、AST[(80.22±4.71)U/L vs (69.26±5.37)U/L],以及肝组织中ROS[(1 030.21±115.87) pg/mL vs (847.84±63.65) pg/mL]、TNFα[(767.18±60.60) ng/mL vs (698.89±16.99) ng/mL]、IL-1β[(29.97±1.37) ng/mL vs (26.70±3.09) ng/mL]、IL-6[(59.13±7.09) pg/mL vs (49.29±3.41) pg/mL)]均显著降低(P值均<0.05);与control组比较,LPS组小鼠肝组织切片HE染色显示出肝小叶结构破坏、炎性细胞浸润、细胞间隙模糊、肝细胞肿胀; LPS+SS-31组炎性细胞浸润减少,肝细胞肿胀减轻。 结论 线粒体靶向抗氧化剂SS-31可降低ROS水平,下调脓毒症时高表达的炎性因子,减轻小鼠脓毒症相关急性肝损伤。 -
关键词:
- 肝疾病 /
- 抗氧化剂 /
- 脓毒症 /
- 小鼠,近交C57BL
Abstract:Objective To investigate the effect of the mitochondria-targeted antioxidant SS-31 on acute liver injury in a mouse model of sepsis. Methods A total of 24 adult male C57BL/6J mice were randomly divided into control group, control+SS-31 group, lipopolysaccharide (LPS) group, and LPS+SS-31 group, with 6 mice in each group. The mice were given intraperitoneal injection of LPS (10 mg/kg) to establish a model of sepsis and acute liver injury, followed by intraperitoneal injection of SS-31 (10 mg/kg) for treatment, and the mice in the control group were given intraperitoneal injection of an equal volume of PBS solution, followed by intraperitoneal injection of an equal volume of normal saline. After 12 hours, ELISA was used to measure the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), reactive oxygen species (ROS), superoxide dismutase (SOD), tumor necrosis factor-α (TNFα), interleukin-1β(IL-1β), and interleukin-6 (IL-6), and HE staining was used to observe liver histopathological changes. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results Compared with the LPS group, the LPS+SS-31 group had significant reductions in the serum levels of ALT (140.05±12.22 U/L vs 102.64±8.75 U/L, P < 0.05) and AST (80.22±4.71 U/L vs 69.26±5.37 U/L, P < 0.05) and the levels of ROS (1 030.21±115.87 pg/mL vs 847.84±63.65 pg/mL, P < 0.05), TNFα (767.18±60.60 ng/mL vs 698.89±16.99 ng/mL, P < 0.05), IL-1β (29.97±1.37 ng/mL vs 26.70±3.09 ng/mL, P < 0.05), and IL-6 (59.13±7.09 pg/mL vs 49.29±3.41 pg/mL, P < 0.05) in liver tissue. Compared with the control group based on HE staining, the LPS group showed destruction of hepatic lobular structure, inflammatory cell infiltration, ambiguous intercellular space, and hepatocyte swelling, while the LPS+SS-31 group showed alleviation of inflammatory cell infiltration and hepatocyte swelling. Conclusion The mitochondria-targeted antioxidant SS-31 can reduce the production of ROS, downregulate the highly expressed inflammatory factors in sepsis, and alleviate sepsis-related acute liver injury in mice. -
Key words:
- Liver Diseases /
- Antioxidants /
- Sepsis /
- Mice, Inbred C57BL
-
表 1 4组小鼠各指标比较
项目 control组
(n=6)control+SS-31组
(n=6)LPS组
(n=6)LPS+SS-31组
(n=6)F值 P值 体质量(g) 20.83±1.68 21.03±1.35 21.53±1.36 20.93±1.37 0.275 0.843 血清指标 ALT(U/L) 73.76±4.72 74.67±8.88 140.05±12.221) 102.64±8.752)3) 71.428 <0.001 AST(U/L) 50.66±1.99 48.75±4.28 80.22±4.711) 69.26±5.372)3) 75.073 <0.001 氧化应激指标 ROS(pg/mL) 704.13±60.08 660.39±84.44 1 030.21±115.871) 847.84±63.652)3) 23.679 <0.001 SOD(pg/mL) 761.84±36.36 736.56±31.15 638.72±22.201) 615.00±46.222) 25.305 <0.001 炎性指标 TNFα(ng/mL) 618.69±56.28 618.95±59.52 767.18±60.601) 698.89±16.992)3) 11.526 <0.001 IL-1β(ng/mL) 23.34±1.05 23.24±0.96 29.97±1.371) 26.70±3.092)3) 18.253 0.001 IL-6(pg/mL) 43.19±7.79 42.96±5.56 59.13±7.091) 49.29±3.412)3) 8.985 0.001 注:与control组比较,1)P<0.05;与control+SS-31组比较,2)P<0.05;与LPS组比较,3)P<0.05。 -
[1] SINGER M, DEUTSCHMAN CS, SEYMOUR CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3)[J]. JAMA, 2016, 315(8): 801-810. DOI: 10.1001/jama.2016.0287. [2] FLEISCHMANN C, SCHERAG A, ADHIKARI NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations[J]. Am J Respir Crit Care Med, 2016, 193(3): 259-272. DOI: 10.1164/rccm.201504-0781OC. [3] WANG L, QIU YL, WANG JS. Advances in research and application of liver organoids[J]. J Clin Hepatol, 2019, 35(10): 2342-2345. DOI: 10.3969/j.issn.1001-5256.2019.10.047.王利, 丘倚灵, 王建设. 肝类器官研究及应用进展[J]. 临床肝胆病杂志, 2019, 35(10): 2342-2345. DOI: 10.3969/j.issn.1001-5256.2019.10.047. [4] WANG DD, SONG J, ZHANG XL. Research advances in the role of gut microbiota in liver diseases[J]. J Clin Hepatol, 2019, 35(9): 2120-2123. DOI: 10.3969/j.issn.1001-5256.2019.09.053.王丹丹, 宋佳, 张晓岚. 肠道菌群在肝脏疾病中的作用[J]. 临床肝胆病杂志, 2019, 35(9): 2120-2123. DOI: 10.3969/j.issn.1001-5256.2019.09.053. [5] XING BM, GUO N, NING HH, et al. Sepsis liver damage and autophagy[J/CD]. Chin J Liver Dis (Electronic Version), 2021, 13(3): 37-41. DOI: 10.3969/j.issn.1674-7380.2021.03.006.邢博民, 郭娜, 宁海慧, 等. 脓毒症肝损伤与自噬[J/CD]. 中国肝脏病杂志(电子版), 2021, 13(3): 37-41. DOI: 10.3969/j.issn.1674-7380.2021.03.006. [6] KOBASHI H, TOSHIMORI J, YAMAMOTO K. Sepsis-associated liver injury: Incidence, classification and the clinical significance[J]. Hepatol Res, 2013, 43(3): 255-266. DOI: 10.1111/j.1872-034X.2012.01069.x. [7] MARSHALL JC. New translational research provides insights into liver dysfunction in sepsis[J]. PLoS Med, 2012, 9(11): e1001341. DOI: 10.1371/journal.pmed.1001341. [8] FUCHS M, SANYAL AJ. Sepsis and cholestasis[J]. Clin Liver Dis, 2008, 12(1): 151-172, ix. DOI: 10.1016/j.cld.2007.11.002. [9] CHIAO YA, ZHANG H, SWEETWYNE M, et al. Late-life restoration of mitochondrial function reverses cardiac dysfunction in old mice[J]. Elife, 2020, 9: e55513. DOI: 10.7554/eLife.55513. [10] YANG L, ZHAO K, CALINGASAN NY, et al. Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine neurotoxicity[J]. Antioxid Redox Signal, 2009, 11(9): 2095-2104. DOI: 10.1089/ars.2009.2445. [11] TALBERT EE, SMUDER AJ, MIN K, et al. Immobilization-induced activation of key proteolytic systems in skeletal muscles is prevented by a mitochondria-targeted antioxidant[J]. J Appl Physiol (1985), 2013, 115(4): 529-538. DOI: 10.1152/japplphysiol.00471.2013. [12] SZETO HH, LIU S, SOONG Y, et al. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury[J]. J Am Soc Nephrol, 2011, 22(6): 1041-1052. DOI: 10.1681/ASN.2010080808. [13] WU J, HAO S, SUN XR, et al. Elamipretide (SS-31) ameliorates isoflurane-induced long-term impairments of mitochondrial morphogenesis and cognition in developing rats[J]. Front Cell Neurosci, 2017, 11: 119. DOI: 10.3389/fncel.2017.00119. [14] ALLEN ME, PENNINGTON ER, PERRY JB, et al. The cardiolipin-binding peptide elamipretide mitigates fragmentation of cristae networks following cardiac ischemia reperfusion in rats[J]. Commun Biol, 2020, 3(1): 389. DOI: 10.1038/s42003-020-1101-3. [15] ZHANG XT, LIU JZ, LI P, et al. Research progress of mitophagy in sepsis[J]. China Med Herald, 2021, 18(10): 39-42. https://www.cnki.com.cn/Article/CJFDTOTAL-YYCY202110011.htm张欣桐, 刘景卓, 李盼, 等. 线粒体自噬在脓毒症中的研究进展[J]. 中国医药导报, 2021, 18(10): 39-42. https://www.cnki.com.cn/Article/CJFDTOTAL-YYCY202110011.htm [16] SUN XD, YAN YH, ZHANG YT, et al. Effects of Xuebijing injection on oxidative stress and early inflammatory factors in rats with sepsis induced liver injury[J]. Chin J Mod Appl Pharm, 2016, 33(10): 1255-1259. DOI: 10.13748/j.cnki.issn1007-7693.2016.10.008.孙雪东, 严一核, 张亦婷, 等. 血必净对脓毒症肝损伤大鼠氧化应激及炎症状态的影响[J]. 中国现代应用药学, 2016, 33(10): 1255-1259. DOI: 10.13748/j.cnki.issn1007-7693.2016.10.008. [17] ZHAO W, XU Z, CAO J, et al. Elamipretide (SS-31) improves mitochondrial dysfunction, synaptic and memory impairment induced by lipopolysaccharide in mice[J]. J Neuroinflammation, 2019, 16(1): 230. DOI: 10.1186/s12974-019-1627-9. [18] SMUDER AJ, ROBERTS BM, WIGGS MP, et al. Pharmacological targeting of mitochondrial function and reactive oxygen species production prevents colon 26 cancer-induced cardiorespiratory muscle weakness[J]. Oncotarget, 2020, 11(38): 3502-3514. DOI: 10.18632/oncotarget.27748. [19] HAO ZH, HUANG Y, WANG MR, et al. SS31 ameliorates age-related activation of NF-κB signaling in senile mice model, SAMP8[J]. Oncotarget, 2017, 8(2): 1983-1992. DOI: 10.18632/oncotarget.14077. [20] BUTLER J, KHAN MS, ANKER SD, et al. Effects of elamipretide on left ventricular function in patients with heart failure with reduced ejection fraction: The PROGRESS-HF phase 2 trial[J]. J Card Fail, 2020, 26(5): 429-437. DOI: 10.1016/j.cardfail.2020.02.001. [21] KARAA A, HAAS R, GOLDSTEIN A, et al. Randomized dose-escalation trial of elamipretide in adults with primary mitochondrial myopathy[J]. Neurology, 2018, 90(14): e1212-e1221. DOI: 10.1212/WNL.0000000000005255. 期刊类型引用(3)
1. 程玉鑫,刘亮,董适毓,李胜超,张萌. 外泌体蛋白、mRNA及非编码RNA调节肝癌发生和发展的研究进展. 实用医学杂志. 2024(06): 748-755 .  百度学术
百度学术2. 王玲,曹信宇,商伟芳,丁秀婷. 慢性乙型肝炎患者外泌体miR-335-5p与肝硬化严重程度的相关性. 中国热带医学. 2024(03): 326-332 .  百度学术
百度学术3. 窦金萍,高维崧,韦双,高新桃,李轶女. 外泌体MicroRNA在抗病毒免疫中的功能分析. 生物技术通报. 2024(05): 48-57 .  百度学术
百度学术其他类型引用(3)
-




 PDF下载 ( 3168 KB)
PDF下载 ( 3168 KB)

 下载:
下载:
 百度学术
百度学术

