Wnt信号通路与肝纤维化的关系
DOI: 10.3969/j.issn.1001-5256.2022.02.038
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:范钦、李红俊、李晓霞、杨尹负责查找文献,资料分析,撰写论文;成家茂、陈海燕负责拟定写作框架,指导思路并修改稿件和最后定稿。
-
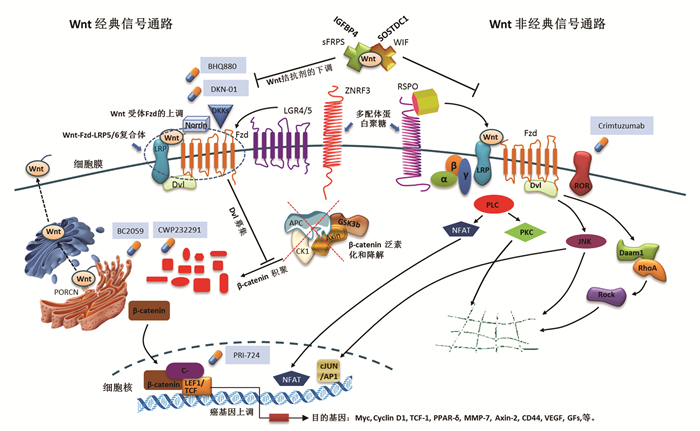
摘要: 在各种慢性肝损伤后,肝纤维化是生物体的一种自我修复性病理过程,并能引起肝硬化、肝癌等疾病。Wnt信号通路广泛存在于无脊椎动物和脊椎动物中,是一类在物种进化过程中高度保守的信号通路。许多研究已经证实Wnt信号通路与肝纤维化的发生发展有密切联系。主要从经典和非经典Wnt信号通路调控肝星状细胞、肝巨噬细胞、肝祖细胞的机制方面进行概述,为后续深入开展Wnt信号调控肝纤维化机制研究及探索可逆转肝纤维化的治疗靶点提供新思路。Abstract: Hepatic fibrosis (HF) is a self-healing pathological process after all kinds of chronic liver injuries and can cause diseases such as liver cirrhosis and liver cancer. The Wnt signaling pathway is highly conserved in species evolution and widely exists in invertebrates and vertebrates, and many studies have confirmed that the Wnt signaling pathway is closely associated with the development and progression of HF. This article reviews the mechanisms of the classical and non-classical Wnt signaling pathways in regulating hepatic stellate cells, hepatic macrophages, and hepatic progenitor cells, so as to provide new ideas for subsequent studies on the mechanism of the Wnt signaling pathway in regulating HF and further exploration of therapeutic targets that can reverse HF.
-
Key words:
- Liver Cirrhosis /
- Wnt Signaling Pathway /
- Hepatic Stellate Cells /
- Kupffer Cells /
- Stem Cells
-
[1] ABOU ZIKI MD, MANI A. The interplay of canonical and noncanonical Wnt signaling in metabolic syndrome[J]. Nutr Res, 2019, 70: 18-25. DOI: 10.1016/j.nutres.2018.06.009. [2] HU HH, CAO G, WU XQ, et al. Wnt signaling pathway in aging-related tissue fibrosis and therapies[J]. Ageing Res Rev, 2020, 60: 101063. DOI: 10.1016/j.arr.2020.101063. [3] FRENQUELLI M, TONON G. WNT signaling in hematological malignancies[J]. Front Oncol, 2020, 10: 615190. DOI: 10.3389/fonc.2020.615190. [4] WANG Y, ZHOU CJ, LIU Y. Wnt signaling in kidney development and disease[J]. Prog Mol Biol Transl Sci, 2018, 153: 181-207. DOI: 10.1016/bs.pmbts.2017.11.019. [5] LI H. Advances in anti hepatic fibrotic therapy with Traditional Chinese Medicine herbal formula[J]. J Ethnopharmacol, 2020, 251: 112442. DOI: 10.1016/j.jep.2019.112442. [6] PERUGORRIA MJ, OLAIZOLA P, LABIANO I, et al. Wnt-β-catenin signalling in liver development, health and disease[J]. Nat Rev Gastroenterol Hepatol, 2019, 16(2): 121-136. DOI: 10.1038/s41575-018-0075-9. [7] KATOH M. Multi-layered prevention and treatment of chronic inflammation, organ fibrosis and cancer associated with canonical WNT/β-catenin signaling activation (Review)[J]. Int J Mol Med, 2018, 42(2): 713-725. DOI: 10.3892/ijmm.2018.3689. [8] PENG JF, WANG ZB, PENG JS, et al. Effect of miR-142-3p targeting Wnt/β-catenin signaling pathway on proliferation of colorectal cancer cells[J]. Clin Misdiagn Misther, 2021, 34(4): 81-86. DOI: 10.3969/j.issn.1002-3429.2021.04.017.彭俊付, 王振彪, 彭继升, 等. miR-142-3p靶向Wnt/β-链蛋白通路对结直肠癌细胞增殖的影响[J]. 临床误诊误治, 2021, 34(4): 81-86. DOI: 10.3969/j.issn.1002-3429.2021.04.017. [9] REN DN, CHEN J, LI Z, et al. LRP5/6 directly bind to Frizzled and prevent Frizzled-regulated tumour metastasis[J]. Nat Commun, 2015, 6: 6906. DOI: 10.1038/ncomms7906. [10] HUA Y, YANG Y, LI Q, et al. Oligomerization of Frizzled and LRP5/6 protein initiates intracellular signaling for the canonical WNT/β-catenin pathway[J]. J Biol Chem, 2018, 293(51): 19710-19724. DOI: 10.1074/jbc.RA118.004434. [11] PASCUAL-CARRERAS E, SUREDA-GÓMEZ M, BARRULL-MASCARÓ R, et al. Wnt-Frizzled-LRP5/6 signaling mediates posterior fate and proliferation during planarian regeneration[J]. Genes (Basel), 2021, 12(1): 101. DOI: 10.3390/genes12010101. [12] XU Q, KRAUSE M, SAMOYLENKO A, et al. Wnt signaling in renal cell carcinoma[J]. Cancers (Basel), 2016, 8(6): 57. DOI: 10.3390/cancers8060057. [13] CHEN Y, CHEN Z, TANG Y, et al. The involvement of noncanonical Wnt signaling in cancers[J]. Biomed Pharmacother, 2021, 133: 110946. DOI: 10.1016/j.biopha.2020.110946. [14] WANG K, XIE D, XIE J, et al. MiR-27a regulates Wnt/beta-catenin signaling through targeting SFRP1 in glioma[J]. Neuroreport, 2015, 26(12): 695-702. DOI: 10.1097/WNR.0000000000000410. [15] HIGASHI T, FRIEDMAN SL, HOSHIDA Y. Hepatic stellate cells as key target in liver fibrosis[J]. Adv Drug Deliv Rev, 2017, 121: 27-42. DOI: 10.1016/j.addr.2017.05.007. [16] GHEORGHE G, BUNGǍU S, CEOBANU G, et al. The non-invasive assessment of hepatic fibrosis[J]. J Formos Med Assoc, 2021, 120(2): 794-803. DOI: 10.1016/j.jfma.2020.08.019. [17] SAEED A, BARTUZI P, HEEGSMA J, et al. Impaired hepatic vitamin A metabolism in NAFLD mice leading to vitamin A accumulation in hepatocytes[J]. Cell Mol Gastroenterol Hepatol, 2021, 11(1): 309-325. DOI: 10.1016/j.jcmgh.2020.07.006. [18] CHEN FY, TU CT. The role and molecular mechanism of Notch signaling transduction pathway on liver fibrogenesis[J/CD]. Chin J Liver Dis (Electronic Version), 2020, 12(4): 23-28. DOI: 10.3969/j.issn.1674-7380.2020.04.004.陈方园, 涂传涛. Notch信号转导通路在肝纤维化形成中的作用与分子机制[J/CD]. 中国肝脏病杂志(电子版), 2020, 12(4): 23-28. DOI: 10.3969/j.issn.1674-7380.2020.04.004. [19] TAO Y, WANG N, QIU T, et al. The role of autophagy and NLRP3 inflammasome in liver fibrosis[J]. Biomed Res Int, 2020, 2020: 7269150. DOI: 10.1155/2020/7269150. [20] KONG Z, LIU R, CHENG Y. Artesunate alleviates liver fibrosis by regulating ferroptosis signaling pathway[J]. Biomed Pharmacother, 2019, 109: 2043-2053. DOI: 10.1016/j.biopha.2018.11.030. [21] TSUKAMOTO H, ZHU NL, WANG J, et al. Morphogens and hepatic stellate cell fate regulation in chronic liver disease[J]. J Gastroenterol Hepatol, 2012, 27(Suppl 2): 94-98. DOI: 10.1111/j.1440-1746.2011.07022.x. [22] MIAO CG, YANG YY, HE X, et al. Wnt signaling in liver fibrosis: Progress, challenges and potential directions[J]. Biochimie, 2013, 95(12): 2326-2335. DOI: 10.1016/j.biochi.2013.09.003. [23] FUNG E, TSUKAMOTO H. Morphogen-related therapeutic targets for liver fibrosis[J]. Clin Res Hepatol Gastroenterol, 2015, 39(Suppl 1): s69-s74. DOI: 10.1016/j.clinre.2015.05.017. [24] CHEN Y, CHEN X, JI YR, et al. PLK1 regulates hepatic stellate cell activation and liver fibrosis through Wnt/β-catenin signalling pathway[J]. J Cell Mol Med, 2020, 24(13): 7405-7416. DOI: 10.1111/jcmm.15356. [25] DU J, REN W, ZHANG Q, et al. Heme oxygenase-1 suppresses Wnt signaling pathway in nonalcoholic steatohepatitis-related liver fibrosis[J]. Biomed Res Int, 2020, 2020: 4910601. DOI: 10.1155/2020/4910601. [26] XU T, PAN L, LI L, et al. MicroRNA-708 modulates hepatic stellate cells activation and enhances extracellular matrix accumulation via direct targeting TMEM88[J]. J Cell Mol Med, 2020, 24(13): 7127-7140. DOI: 10.1111/jcmm.15119. [27] YANG J, TAO Q, ZHOU Y, et al. MicroRNA-708 represses hepatic stellate cells activation and proliferation by targeting ZEB1 through Wnt/β-catenin pathway[J]. Eur J Pharmacol, 2020, 871: 172927. DOI: 10.1016/j.ejphar.2020.172927. [28] PAN Q, GUO CJ, XU QY, et al. miR-16 integrates signal pathways in myofibroblasts: Determinant of cell fate necessary for fibrosis resolution[J]. Cell Death Dis, 2020, 11(8): 639. DOI: 10.1038/s41419-020-02832-z. [29] WANG Y, FAN X, LEI N, et al. A microRNA derived from schistosoma japonicum promotes schistosomiasis hepatic fibrosis by targeting host secreted frizzled-related protein 1[J]. Front Cell Infect Microbiol, 2020, 10: 101. DOI: 10.3389/fcimb.2020.00101. [30] WANG L, LIAO Y, YANG R, et al. Sja-miR-71a in Schistosome egg-derived extracellular vesicles suppresses liver fibrosis caused by schistosomiasis via targeting semaphorin 4D[J]. J Extracell Vesicles, 2020, 9(1): 1785738. DOI: 10.1080/20013078.2020.1785738. [31] ZHANG M, HAUGHEY M, WANG NY, et al. Targeting the Wnt signaling pathway through R-spondin 3 identifies an anti-fibrosis treatment strategy for multiple organs[J]. PLoS One, 2020, 15(3): e0229445. DOI: 10.1371/journal.pone.0229445. [32] YU HX, YAO Y, BU FT, et al. Blockade of YAP alleviates hepatic fibrosis through accelerating apoptosis and reversion of activated hepatic stellate cells[J]. Mol Immunol, 2019, 107: 29-40. DOI: 10.1016/j.molimm.2019.01.004. [33] ELDEEB MK, MAGOUR GM, BEDAIR RN, et al. Study of Dickkopf-1 (DKK-1) in patients with chronic viral hepatitis C-related liver cirrhosis with and without hepatocellular carcinoma[J]. Clin Exp Hepatol, 2020, 6(2): 85-91. DOI: 10.5114/ceh.2020.95831. [34] KIM DH, KIM EJ, KIM DH, et al. Dact2 is involved in the regulation of epithelial-mesenchymal transition[J]. Biochem Biophys Res Commun, 2020, 524(1): 190-197. DOI: 10.1016/j.bbrc.2019.12.090. [35] LI LY, YANG JF, RONG F, et al. ZEB1 serves an oncogenic role in the tumourigenesis of HCC by promoting cell proliferation, migration, and inhibiting apoptosis via Wnt/β-catenin signaling pathway[J]. Acta Pharmacol Sin, 2021. DOI: 10.1038/s41401-020-00575-3.[Online ahead of print] [36] SHAN Z, JU C. Hepatic macrophages in liver injury[J]. Front Immunol, 2020, 11: 322. DOI: 10.3389/fimmu.2020.00322. [37] ZENG G, AWAN F, OTRUBA W, et al. Wnt'er in liver: Expression of Wnt and frizzled genes in mouse[J]. Hepatology, 2007, 45(1): 195-204. DOI: 10.1002/hep.21473. [38] FENG Y, LIANG Y, ZHU X, et al. The signaling protein Wnt5a promotes TGFβ1-mediated macrophage polarization and kidney fibrosis by inducing the transcriptional regulators Yap/Taz[J]. J Biol Chem, 2018, 293(50): 19290-19302. DOI: 10.1074/jbc.RA118.005457. [39] HOU J, SHI J, CHEN L, et al. M2 macrophages promote myofibroblast differentiation of LR-MSCs and are associated with pulmonary fibrogenesis[J]. Cell Commun Signal, 2018, 16(1): 89. DOI: 10.1186/s12964-018-0300-8. [40] YANG Y, YE YC, CHEN Y, et al. Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors[J]. Cell Death Dis, 2018, 9(8): 793. DOI: 10.1038/s41419-018-0818-0. [41] TIAN X, WU Y, YANG Y, et al. Long noncoding RNA LINC00662 promotes M2 macrophage polarization and hepatocellular carcinoma progression via activating Wnt/β-catenin signaling[J]. Mol Oncol, 2020, 14(2): 462-483. DOI: 10.1002/1878-0261.12606. [42] JIANG Y, HAN Q, ZHAO H, et al. Promotion of epithelial-mesenchymal transformation by hepatocellular carcinoma-educated macrophages through Wnt2b/β-catenin/c-Myc signaling and reprogramming glycolysis[J]. J Exp Clin Cancer Res, 2021, 40(1): 13. DOI: 10.1186/s13046-020-01808-3. [43] CORBETT L, MANN J, MANN DA. Non-canonical Wnt predominates in activated rat hepatic stellate cells, influencing HSC survival and paracrine stimulation of Kupffer cells[J]. PLoS One, 2015, 10(11): e0142794. DOI: 10.1371/journal.pone.0142794. [44] AKCORA BÖ, STORM G, BANSAL R. Inhibition of canonical WNT signaling pathway by β-catenin/CBP inhibitor ICG-001 ameliorates liver fibrosis in vivo through suppression of stromal CXCL12[J]. Biochim Biophys Acta Mol Basis Dis, 2018, 1864(3): 804-818. DOI: 10.1016/j.bbadis.2017.12.001. [45] KAKINUMA S, KAMIYA A. A rodent model for cell transplantation of hepatic progenitor cells[J]. Methods Mol Biol, 2019, 1905: 211-219. DOI: 10.1007/978-1-4939-8961-4_19. [46] PINHEIRO D, DIAS I, RIBEIRO SILVA K, et al. Mechanisms underlying cell therapy in liver fibrosis: An overview[J]. Cells, 2019, 8(11): 1339. DOI: 10.3390/cells8111339. [47] DAI Z, SONG G, BALAKRISHNAN A, et al. Growth differentiation factor 11 attenuates liver fibrosis via expansion of liver progenitor cells[J]. Gut, 2020, 69(6): 1104-1115. DOI: 10.1136/gutjnl-2019-318812. [48] BOULTER L, GOVAERE O, BIRD TG, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease[J]. Nat Med, 2012, 18(4): 572-579. DOI: 10.1038/nm.2667. [49] CARPINO G, NOBILI V, RENZI A, et al. Macrophage activation in pediatric nonalcoholic fatty liver disease (NAFLD) correlates with hepatic progenitor cell response via Wnt3a pathway[J]. PLoS One, 2016, 11(6): e0157246. DOI: 10.1371/journal.pone.0157246. [50] MA Z, LI F, CHEN L, et al. Autophagy promotes hepatic differentiation of hepatic progenitor cells by regulating the Wnt/β-catenin signaling pathway[J]. J Mol Histol, 2019, 50(1): 75-90. DOI: 10.1007/s10735-018-9808-x. [51] ZENG J, JING Y, WU Q, et al. Autophagy is required for hepatic differentiation of hepatic progenitor cells via Wnt signaling pathway[J]. Biomed Res Int, 2021, 2021: 6627506. DOI: 10.1155/2021/6627506. -



 PDF下载 ( 2748 KB)
PDF下载 ( 2748 KB)

 下载:
下载:


