原发性肝癌高危人群的早期预警和精准筛查
DOI: 10.3969/j.issn.1001-5256.2022.03.002
利益冲突声明: 所有作者均声明不存在利益冲突。
作者贡献声明: 郝新负责撰写论文; 樊蓉、侯金林负责拟定写作思路,指导撰写、修改文章并最后定稿。
Early warning and accurate screening for the high-risk population of hepatocellular carcinoma
-
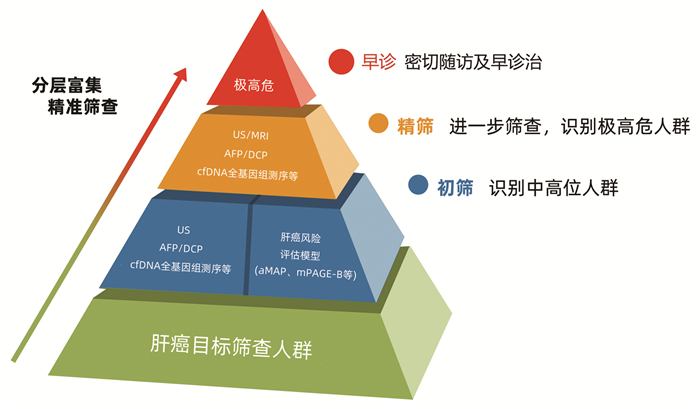
摘要: 对我国慢性肝病人群开展规范筛查,实现肝癌危险人群精准分层与规范管理,并通过有效技术手段早期预警肝癌,是提升我国肝癌早期诊断率的有效方法。临床实践中可以利用aMAP等肝癌风险评分模型对慢性肝病人群进行风险初筛,识别罹患肝癌中、高危人群,通过新型血清标志物进一步鉴定出肝癌极高危人群,并应用影像学技术早诊筛查。通过分层富集,不断探索、完善肝癌早筛早诊“金字塔”筛查管理模式,以最终实现提高肝癌早期诊断率并降低肝癌相关病死率的目标。Abstract: It is an effective way to improve the early diagnosis rate of hepatocellular carcinoma (HCC) in China by conducting standardized screening among the population with chronic liver diseases, achieving accurate stratification and standardized management of the population at a risk of HCC, and realizing the early warning for HCC through effective technical means. In clinical practice, the risk scoring models for liver cancer including aMAP can be used for preliminary risk screening of the population with chronic liver diseases to identify the population with a moderate or high risk of liver cancer. Novel serum biomarkers can be used to further screen out the population with an extremely high risk of liver cancer, and then imaging technology can be used for early diagnosis. Constant exploration and improvement of the "pyramid" screening and management mode through stratified enrichment may help to achieve the goal of improving early diagnosis rate of HCC and reducing HCC-related mortality.
-
Key words:
- Key words: Carcinoma, Hepatocellular /
- Screening /
- Early Diagnosis
-
[1] International Agency for Research on Cancer, World Health Organization. Liver Source, Globocan 2020[EB/OL]. http://gco.iarc.fr/today//data/factsheets/cancers/11-Liver-fact-sheet.pdf. [2] PARK JW, CHEN M, COLOMBO M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study[J]. Liver Int, 2015, 35(9): 2155-2166. DOI: 10.1111/liv.12818. [3] ALLEMANI C, MATSUDA T, di CARLO V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries[J]. Lancet, 2018, 391(10125): 1023-1075. DOI: 10.1016/S0140-6736(17)33326-3. [4] European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma[J]. J Hepatol, 2018, 69(1): 182-236. DOI: 10.1016/j.jhep.2018.03.019. [5] KUDO M. Management of hepatocellular carcinoma in Japan as a world-leading model[J]. Liver Cancer, 2018, 7(2): 134-147. DOI: 10.1159/000484619. [6] KUDO M, IZUMI N, ICHIDA T, et al. Report of the 19th follow-up survey of primary liver cancer in Japan[J]. Hepatol Res, 2016, 46(5): 372-390. DOI: 10.1111/hepr.12697. [7] BRUIX J, QIN S, MERLE P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet, 2017, 389(10064): 56-66. DOI: 10.1016/S0140-6736(16)32453-9. [8] SINGAL AG, PILLAI A, TIRO J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: A meta-analysis[J]. PLoS Med, 2014, 11(4): e1001624. DOI: 10.1371/journal.pmed.1001624. [9] Bureau of Medical Administration, National Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007.中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗规范(2019年版)[J]. 临床肝胆病杂志, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. [10] Chinese Society of Clinical Oncology. Guidelines for diagnosis and treatment of primary liver cancer (2020)[M]. Beijing: People's Health Publishing House, 2020.中国临床肿瘤学会. 原发性肝癌诊疗指南(2020)[M]. 北京: 人民卫生出版社, 2020. [11] National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association, Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: A 2018 update[J]. J Clin Hepatol, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007.中华医学会肝病学分会脂肪肝和酒精性肝病学组, 中国医师协会脂肪性肝病专家委员会. 非酒精性脂肪性肝病防治指南(2018年更新版)[J]. 临床肝胆病杂志, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007. [12] FORNER A, REIG M, BRUIX J. Hepatocellular carcinoma[J]. Lancet, 2018, 391(10127): 1301-1314. DOI: 10.1016/S0140-6736(18)30010-2. [13] WANG M, WANG Y, FENG X, et al. Contribution of hepatitis B virus and hepatitis C virus to liver cancer in China north areas: Experience of the Chinese National Cancer Center[J]. Int J Infect Dis, 2017, 65: 15-21. DOI: 10.1016/j.ijid.2017.09.003. [14] Global Burden of Disease Liver Cancer Collaboration, AKINYEMIJU T, ABERA S, et al. The Burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the Global Burden of Disease Study 2015[J]. JAMA Oncol, 2017, 3(12): 1683-1691. DOI: 10.1001/jamaoncol.2017.3055. [15] Chinese Society of Hepatology, Chinese Medical Association. Consensus on the secondary prevention for primary liver cancer (2021 edition)[J]. J Clin Hepatol, 2021, 37(3): 532-542. DOI: 10.3969/j.issn.1001-5256.2021.03.008.中华医学会肝病学分会. 原发性肝癌二级预防共识(2021年版)[J]. 临床肝胆病杂志, 2021, 37(3): 532-542. DOI: 10.3969/j.issn.1001-5256.2021.03.008. [16] LOK AS, MCMAHON BJ, BROWN RS Jr, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis[J]. Hepatology, 2016, 63(1): 284-306. DOI: 10.1002/hep.28280. [17] MU XM, WANG W, JIANG YY, et al. Patterns of comorbidity in hepatocellular carcinoma: A network perspective[J]. Int J Environ Res Public Health, 2020, 17(9): 3108. DOI: 10.3390/ijerph17093108. [18] Professional Committee for Prevention and Control of Hepatobiliary and Pancreatic Diseases of Chinese Preventive Medicine Association; Professional Committee for Hepatology, Chinese Research Hospital Association; Chinese Society of Hepatology, Chinese Medical Association, et al. Guideline for stratified screening and surveillance of primary liver cancer (2020 edition)[J]. J Clin Hepatol, 2021, 37(2): 286-295. DOI: 10.3969/j.issn.1001-5256.2021.02.009.中华预防医学会肝胆胰疾病预防与控制专业委员会, 中国研究型医院学会肝病专业委员会, 中华医学会肝病学分会, 等. 原发性肝癌的分层筛查与监测指南(2020版)[J]. 临床肝胆病杂志, 2021, 37(2): 286-295. DOI: 10.3969/j.issn.1001-5256.2021.02.009. [19] SHARMA SA, KOWGIER M, HANSEN BE, et al. Toronto HCC risk index: A validated scoring system to predict 10-year risk of HCC in patients with cirrhosis[J]. J Hepatol, 2017. DOI: 10.1016/j.jhep.2017.07.033. [20] ZHANG H, ZHU J, XI L, et al. Validation of the Toronto hepatocellular carcinoma risk index for patients with cirrhosis in China: A retrospective cohort study[J]. World J Surg Oncol, 2019, 17(1): 75. DOI: 10.1186/s12957-019-1619-3. [21] YANG HI, YUEN MF, CHAN HL, et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): Development and validation of a predictive score[J]. Lancet Oncol, 2011, 12(6): 568-574. DOI: 10.1016/S1470-2045(11)70077-8. [22] FAN C, LI M, GAN Y, et al. A simple AGED score for risk classification of primary liver cancer: Development and validation with long-term prospective HBsAg-positive cohorts in Qidong, China[J]. Gut, 2019, 68(5): 948-949. DOI: 10.1136/gutjnl-2018-316525. [23] PAPATHEODORIDIS G, DALEKOS G, SYPSA V, et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy[J]. J Hepatol, 2016, 64(4): 800-806. DOI: 10.1016/j.jhep.2015.11.035. [24] YIP TC, WONG GL, WONG VW, et al. Reassessing the accuracy of PAGE-B-related scores to predict hepatocellular carcinoma development in patients with chronic hepatitis B[J]. J Hepatol, 2020, 72(5): 847-854. DOI: 10.1016/j.jhep.2019.12.005. [25] FAN R, PAPATHEODORIDIS G, SUN J, et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis[J]. J Hepatol, 2020, 73(6): 1368-1378. DOI: 10.1016/j.jhep.2020.07.025. [26] SHIHA G, MIKHAIL N, SOLIMAN R. External validation of aMAP risk score in patients with chronic hepatitis C genotype 4 and cirrhosis who achieved SVR following DAAs[J]. J Hepatol, 2021, 74(4): 994-996. DOI: 10.1016/j.jhep.2020.10.008. [27] LI XH, HAO X, DENG YH, et al. Application of aMAP score to assess the risk of hepatocarciongenesis in population of chronic liver disease in primary hospitals[J]. Chin J Hepatol, 2021, 29(4): 6. DOI: 10.3760/cma.j.cn501113-20210329-00144.李秀华, 郝新, 邓永红, 等. 应用aMAP评分评估基层医院慢性肝病人群的肝癌发生风险[J]. 中华肝脏病杂志, 2021, 29(4): 6. DOI: 10.3760/cma.j.cn501113-20210329-00144. [28] RENZULLI M, BISELLI M, BROCCHI S, et al. New hallmark of hepatocellular carcinoma, early hepatocellular carcinoma and high-grade dysplastic nodules on Gd-EOB-DTPA MRI in patients with cirrhosis: A new diagnostic algorithm[J]. Gut, 2018, 67(9): 1674-1682. DOI: 10.1136/gutjnl-2017-315384. [29] ZENG MS, YE HY, GUO L, et al. Gd-EOB-DTPA-enhanced magnetic resonance imaging for focal liver lesions in Chinese patients: A multicenter, open-label, phase Ⅲ study[J]. Hepatobiliary Pancreat Dis Int, 2013, 12(6): 607-616. DOI: 10.1016/s1499-3872(13)60096-x. [30] ICHIKAWA T, SAITO K, YOSHIOKA N, et al. Detection and characterization of focal liver lesions: A Japanese phase Ⅲ, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease[J]. Invest Radiol, 2010, 45(3): 133-141. DOI: 10.1097/RLI.0b013e3181caea5b. [31] Prospective suRveillance for very Early hepatoCellular cARcinoma (PreCar) expert panel. Expert consensus on early screening strategies for liver cancer in China[J]. Chin Hepatol, 2021, 26(8): 825-831. DOI: 10.3969/j.issn.1008-1704.2021.08.001.全国多中心前瞻性肝癌极早期预警筛查项目(PreCar)专家组. 中国肝癌早筛策略专家共识[J]. 肝脏, 2021, 26(8): 825-831. DOI: 10.3969/j.issn.1008-1704.2021.08.001. [32] CHEN L, ABOU-ALFA GK, ZHENG B, et al. Genome-scale profiling of circulating cell-free DNA signatures for early detection of hepatocellular carcinoma in cirrhotic patients[J]. Cell Res, 2021, 31(5): 589-592. DOI: 10.1038/s41422-020-00457-7. [33] HARRIS PS, HANSEN RM, GRAY ME, et al. Hepatocellular carcinoma surveillance: An evidence-based approach[J]. World J Gastroenterol, 2019, 25(13): 1550-1559. DOI: 10.3748/wjg.v25.i13.1550. [34] ATIQ O, TIRO J, YOPP AC, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis[J]. Hepatology, 2017, 65(4): 1196-1205. DOI: 10.1002/hep.28895. [35] YI X, YU S, BAO Y. Alpha-fetoprotein-L3 in hepatocellular carcinoma: A meta-analysis[J]. Clin Chim Acta, 2013, 425: 212-220. DOI: 10.1016/j.cca.2013.08.005. [36] ZHOU JM, WANG T, ZHANG KH. AFP-L3 for the diagnosis of early hepatocellular carcinoma: A meta-analysis[J]. Medicine (Baltimore), 2021, 100(43): e27673. DOI: 10.1097/MD.0000000000027673. [37] XING H, ZHENG YJ, HAN J, et al. Protein induced by vitamin K absence or antagonist-Ⅱ versus alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: A systematic review with meta-analysis[J]. Hepatobiliary Pancreat Dis Int, 2018, 17(6): 487-495. DOI: 10.1016/j.hbpd.2018.09.009. [38] JI J, WANG H, LI Y, et al. Diagnostic evaluation of des-gamma-carboxy prothrombin versus α-fetoprotein for hepatitis B virus-related hepatocellular carcinoma in China: A large-scale, multicentre study[J]. PLoS One, 2016, 11(4): e0153227. DOI: 10.1371/journal.pone.0153227. [39] BEST J, BILGI H, HEIDER D, et al. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma[J]. Z Gastroenterol, 2016, 54(12): 1296-1305. DOI: 10.1055/s-0042-119529. [40] BERHANE S, TOYODA H, TADA T, et al. Role of the GALAD and BALAD-2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients[J]. Clin Gastroenterol Hepatol, 2016, 14(6): 875-886. e6. DOI: 10.1016/j.cgh.2015.12.042. [41] REBOUISSOU S, NAULT JC. Advances in molecular classification and precision oncology in hepatocellular carcinoma[J]. J Hepatol, 2020, 72(2): 215-229. DOI: 10.1016/j.jhep.2019.08.017. [42] SHIGEYASU K, TODEN S, ZUMWALT TJ, et al. Emerging role of microRNAs as liquid biopsy biomarkers in gastrointestinal cancers[J]. Clin Cancer Res, 2017, 23(10): 2391-2399. DOI: 10.1158/1078-0432.CCR-16-1676. [43] CROWLEY E, DI NICOLANTONIO F, LOUPAKIS F, et al. Liquid biopsy: Monitoring cancer-genetics in the blood[J]. Nat Rev Clin Oncol, 2013, 10(8): 472-484. DOI: 10.1038/nrclinonc.2013.110. [44] QU C, WANG Y, WANG P, et al. Detection of early-stage hepatocellular carcinoma in asymptomatic HBsAg-seropositive individuals by liquid biopsy[J]. Proc Natl Acad Sci U S A, 2019, 116(13): 6308-6312. DOI: 10.1073/pnas.1819799116. [45] XU RH, WEI W, KRAWCZYK M, et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma[J]. Nat Mater, 2017, 16(11): 1155-1161. DOI: 10.1038/nmat4997. [46] CHALASANI NP, BHATTACHARYA A, BOOK A, et al. Algorithm for bloodbased panel of methylated DNA and protein markers to detect early-stage hepatocellular carcinoma with high specifificity[J]. J Clin Oncol, 2020, 38(15_suppl): 4577. DOI: 10.1200/JCO.2020.38.15_ suppl.4577. [47] ZHOU J, YU L, GAO X, et al. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma[J]. J Clin Oncol, 2011, 29(36): 4781-4788. DOI: 10.1200/JCO.2011.38.2697. -



 PDF下载 ( 2121 KB)
PDF下载 ( 2121 KB)

 下载:
下载:


