舒肝宁注射液对于氯丙嗪引起的新型组织工程肝构建的胆汁淤积型药物性肝损伤模型的影响
DOI: 10.3969/j.issn.1001-5256.2022.03.018
伦理学声明:本研究方案于2020年6月5日经由北京佑安医院实验动物伦理委员会审批通过,批号:AEEI-2020-076,符合实验室动物管理与使用准则。
利益冲突声明:本研究不存在研究者、伦理委员会成员、受试者监护人以及与公开研究成果有关的利益冲突,特此声明。
作者贡献声明:黄龙负责撰写论文;吴桥负责课题设计,修改论文;陈煜、段钟平负责提供写作思路,指导撰写文章并最后定稿。
Effect of Shuganning injection on the model of cholestasis - type chlorpromazine - induced liver injury constructed by a new tissue - engineered liver
-
摘要:
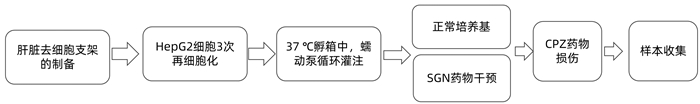
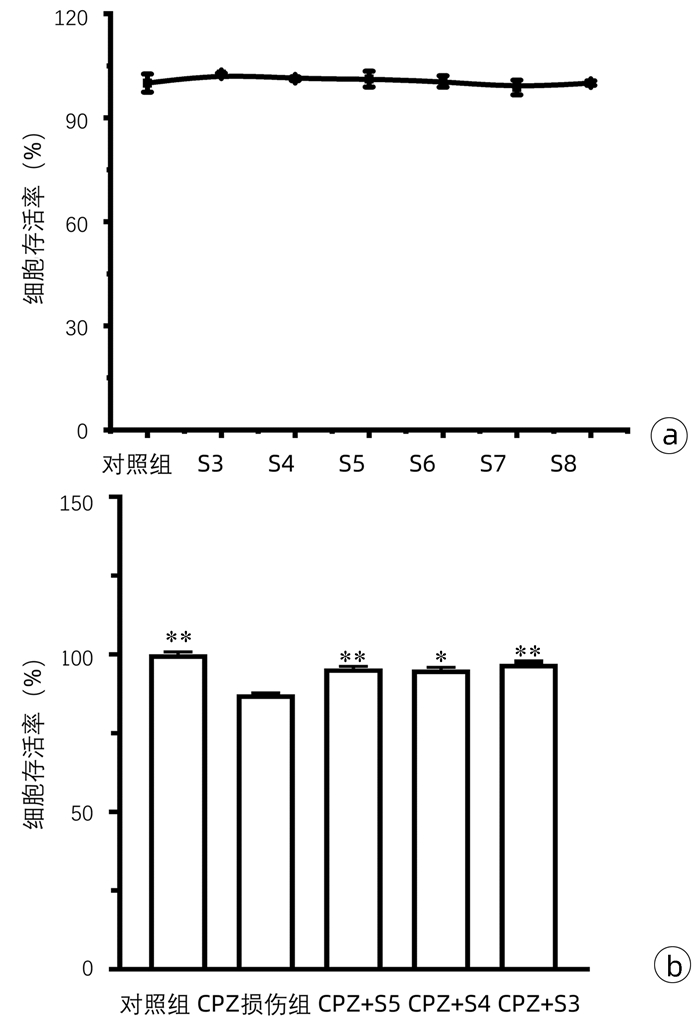
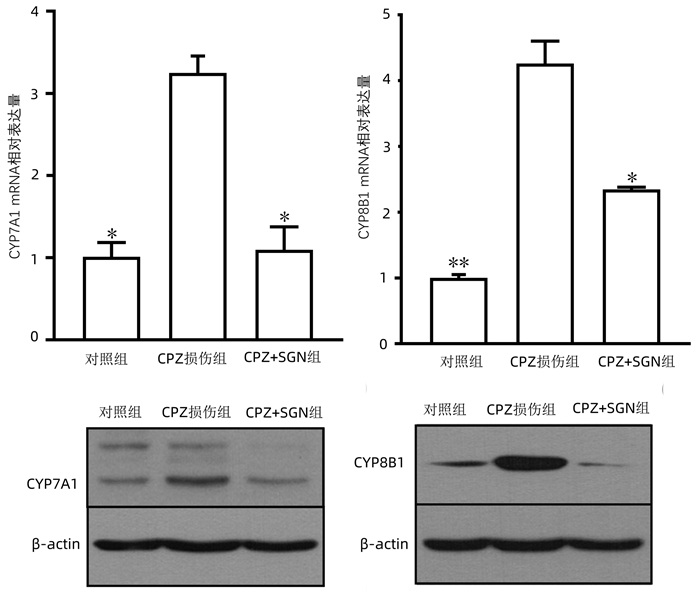
目的 研究舒肝宁注射液(SGN)减轻药物引起的胆汁淤积症以及可能涉及的机制。 方法 将Sprague-Dawley大鼠肝脏去细胞化制为肝脏胶原支架,用人HepG2细胞对支架再细胞化,从而获得组织工程肝(正常对照组),加入10 μmol/L浓度的氯丙嗪(CPZ)与胆汁酸盐混合物灌注组织工程肝建立药物性胆汁淤积模型(CPZ损伤组),该模型进一步用舒肝宁注射液(103倍稀释)处理后为损伤保护组(CPZ+SGN)。随后检测不同组别的肝细胞损伤标志物(ALT、AST、LDH、ALP)、抗氧化及氧化应激标志物(GSH、MDA、SOD、ROS),比较正常组、CPZ组和SGN组肝脏胆汁酸盐代谢相关酶类mRNA以及Western Blot表达水平; 比较正常组、CPZ组和SGN组肝脏胆汁淤积相关酶类mRNA以及Western blot表达水平。并行HE染色评估肝脏病理学。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 CPZ+SGN组ALT、AST、LDH、ALP肝细胞损伤标记指标较CPZ损伤组显著降低(P值均 < 0.000 1),CPZ+SGN组GSH、SOD氧化应激标志物较CPZ组升高(P值分别为 < 0.000 1、 < 0.001),MDA和ROS指标较CPZ组降低(P值分别为 < 0.000 1、 < 0.001)。CPZ+SGN组较CPZ组肝细胞的胆固醇7α-羟化酶、胆固醇12α羟化酶的mRNA表达明显降低(P值均 < 0.001),法尼醇X受体、小异二聚体伴侣、胆盐输出泵及和多药耐药相关蛋白2的mRNA表达水平显著升高(P值分别为 < 0.000 1、 < 0.01、 < 0.000 1、 < 0.000 1)。HE染色结果显示CPZ+SGN组肝细胞的损伤明显减少,细胞的数量相对于损伤组有明显的提高。 结论 SGN可以减轻CPZ引起的药物性胆汁淤积肝损伤,其主要保护机制是通过激活肝细胞的法尼醇X受体,增加小异二聚体伴侣表达发挥调控胆汁酸盐平衡的保护作用,同时抑制胆固醇7α-羟化酶和胆固醇12α羟化酶等减少疏水性胆汁酸合成,上调胆盐输出泵、多药耐药相关蛋白2的表达促进胆汁酸盐的排出。 -
关键词:
- 化学性与药物性肝损伤 /
- 胆汁淤积 /
- 舒肝宁注射液 /
- 大鼠, Sprague-Dawley
Abstract:Objective To investigate the effect of Shuganning injection (SGN) in alleviating drug-induced cholestasis and the possible mechanisms involved. Methods The liver of Sprague-Dawley rats was decellularized to prepare collagen scaffolds, and then the scaffolds were recellularized with human HepG2 cells to obtain the tissue-engineered liver (normal control group). The tissue-engineered liver was perfused with 10 μmol/L chlorpromazine (CPZ) and bile salt mixture to establish a model of drug-induced cholestasis (CPZ group), and the model was further treated with Shuganning injection (103-fold dilution) as the injury protection group (SGN+CPZ group). The markers for hepatocellular injury [alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and alkaline phosphatase (ALP)] and the antioxidant and oxidative stress markers [glutathione (GSH), malondialdehyde (MDA), superoxide dismutase (SOD), and reactive oxygen species (ROS)] were measured for all groups, and the normal control group, the CPZ group, and the SGN+CPZ group were compared in terms of the mRNA and protein expression levels of the enzymes associated with liver bile salt metabolism and the enzymes associated with hepatic cholestasis. HE staining was performed to observe liver pathology. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results Compared with the CPZ group, the SGN+CPZ group had significant reductions in the markers for hepatocellular injury ALT, AST, LDH, and ALP (all P < 0.000 1), significant increases in the oxidative stress markers GSH and SOD (P < 0.000 1 and P < 0.001), and significant reductions in the markers MDA and ROS (P < 0.000 1 and P < 0.001). Compared with the CPZ group, the SGN+CPZ group had significant reductions in the mRNA expression levels of cholesterol 7α-hydroxylase (CYP7A1) and sterol 12α-hydroxylase (CPY8B1) in hepatocytes (all P < 0.001) and significant increases in the mRNA expression levels of farnesoid X receptor (FXR), small heterodimeric partner (SHP), bile salt export pump (BSEP), and multidrug resistance-associated protein 2 (MRP2) (P < 0.000 1, P < 0.01, P < 0.000 1, and P < 0.000 1). HE staining showed that compared with the CPZ group, the SGN+CPZ group had a significant reduction in hepatocyte injury and a significant increase in the number of cells. Conclusion Shuganning injection can alleviate drug-induced cholestatic liver injury caused by chlorpromazine, and it exerts a protective effect by activating FXR in hepatocytes and increasing the expression of SHP to regulate bile salt balance. It also inhibits CYP7A1 and CYP8B1 to reduce the synthesis of hydrophobic bile acids and upregulates the expression of BSEP and MRP2 to promote the excretion of bile salts. -
表 1 1000×的混合胆汁酸盐配方表
混合胆汁酸盐 体积 胆酸 410 μL 鹅去氧胆酸 640 μL 脱氧胆酸 480 μL 石胆酸 8 μL 熊去氧胆酸 14 μL 甘氨鹅脱氧胆酸 160 μL 总计 1712 μL 表 2 PCR引物序列
引物名称 序列(5′-3′) GAPDH F GGACTCATGACCACAGTCCA R TCAGCTCAGGGATGACCTTG SHP F CTCGGTTTGCATACAGTGTTTGAC R GCATATTGGCCTGGAGGTTTT CYP7A1 F CAGAACTGAATGACCTGCCA R GGTGCAAAGTGAAATCCTCC CYP8B1 F ATGAAGGCTGTGCGAGAG R TCTCTTCCATCACGCTGTC BSEP F TGAGCCTGGTCATCTTGTG R TCCGTAAATATTGGCTTTCTG MRP2 F ACAGAGGCTGGTGGCAACC R ACCATTACCTTGTCACTGTCCATGA FXR F ACTGACCTGTGAGGGGTGTA R TGCCCCCGTTTTTACACTTG 注:CYP7A1,胆固醇7α-羟化酶;CYP8B1,甾醇12α-羟化酶;SHP,小异二聚体伴侣。 -
[1] SHEN T, HUANG X, WANG YY, et al. Current status of epidemiological studies on drug-induced liver injury in China[J]. J Clin Hepatol, 2018, 34(6): 1152-1155. DOI: 10.3969/j.issn. 1001-5256.2018.06.002.沈弢, 黄昕, 王誉雅, 等. 我国药物性肝损伤流行病学研究现状[J]. 临床肝胆病杂志, 2018, 34(6): 1152-1155. DOI: 10.3969/j.issn.1001-5256.2018.06.002. [2] Drug-induced Liver Disease Study Group, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the management of drug-induced liver injury[J]. J Clin Hepatol, 2015, 31(11): 1752-1769. DOI: 10.3969/j.issn.1001-5256.2015.11.002.中华医学会肝病学分会药物性肝病学组. 药物性肝损伤诊治指南[J]. 临床肝胆病杂志, 2015, 31(11): 1752-1769. DOI: 10.3969/j.issn.1001-5256.2015.11.002. [3] ZENG LR, HUANG XM, YANG CY, et al. Safety, rationality and drug use of Shuganning injection[J]. Chin J Clin Ration Drug Use, 2021, 14(15): 35-37, 41. DOI: 10.15887/j.cnki.13-1389/r.2021.15.012.曾令荣, 黄雪梅, 杨传玉, 等. 舒肝宁注射液临床应用安全性、合理性及药物利用情况分析[J]. 临床合理用药杂志, 2021, 14(15): 35-37, 41. DOI: 10.15887/j.cnki.13-1389/r.2021.15.012. [4] HENDRIKS DF, FREDRIKSSON PUIGVERT L, MESSNER S, et al. Hepatic 3D spheroid models for the detection and study of compounds with cholestatic liability[J]. Sci Rep, 2016, 6: 35434. DOI: 10.1038/srep35434. [5] DU LN, YANG Y. Establishment and application of animal models of cholestasis[J]. J Clin Hepatol, 2019, 35(2): 444-447. DOI: 10.3969/j.issn.1001-5256.2019.02.046.杜丽娜, 杨燕. 胆汁淤积动物模型的构建及应用前景[J]. 临床肝胆病杂志, 2019, 35(2): 444-447. DOI: 10.3969/j.issn.1001-5256.2019.02.046. [6] ANTHÉRIEU S, BACHOUR-EL AZZI P, DUMONT J, et al. Oxidative stress plays a major role in chlorpromazine-induced cholestasis in human HepaRG cells[J]. Hepatology, 2013, 57(4): 1518-1529. DOI: 10.1002/hep.26160. [7] COPPLE BL, JAESCHKE H, KLAASSEN CD. Oxidative stress and the pathogenesis of cholestasis[J]. Semin Liver Dis, 2010, 30(2): 195-204. DOI: 10.1055/s-0030-1253228. [8] CHATTERJEE S, ANNAERT P. Drug-induced cholestasis: Mechanisms, models, and markers[J]. Curr Drug Metab, 2018, 19(10): 808-818. DOI: 10.2174/1389200219666180427165035. [9] AI G, YAN YD, HUANG ZM. Research progress on Chinese materia medica in treatment of intrahepatic cholestasis based on FXR-mediated regulation[J]. Chin Tradit Herb Drug, 2017, 48(19): 4077-4088. DOI: 10.7501/j.issn.0253-2670.2017.19.028.艾国, 颜耀东, 黄正明. 基于调控法尼醇X受体的中药治疗肝内胆汁瘀积的研究进展[J]. 中草药, 2017, 48(19): 4077-4088. DOI: 10.7501/j.issn.0253-2670.2017.19.028. [10] LIU YQ, CAI X, QIN RW, et al. Regulation effect of leech on farnesol X receptor pathway in rat model of intrahepatic cholestasis[J]. China Med Herald, 2021, 18(5): 4-9. https://www.cnki.com.cn/Article/CJFDTOTAL-YYCY202105003.htm刘玉青, 蔡昕, 覃仁武, 等. 水蛭对肝内胆汁淤积大鼠模型法尼醇X受体通路的调节作用[J]. 中国医药导报, 2021, 18(5): 4-9. https://www.cnki.com.cn/Article/CJFDTOTAL-YYCY202105003.htm [11] LI YX, LI XP, GU J, et al. Study on protective mechanism of Tibetan medicine Ershiwuwei Songshi Pills on cholestatic liver injury in rats based on FXR signaling pathway[J]. China J Chin Mater Med, 2020, 45(21): 5273-5279. DOI: 10.19540/j.cnki.cjcmm.20200727.401.李彦希, 李晓朋, 顾健, 等. 基于FXR信号通路研究藏族药二十五味松石丸对胆汁淤积型肝损伤大鼠的保护作用机制[J]. 中国中药杂志, 2020, 45(21): 5273-5279. DOI: 10.19540/j.cnki.cjcmm.20200727.401. [12] LU TT, REPA JJ, MANGELSDORF DJ. Orphan nuclear receptors as eLiXiRs and FiXeRs of sterol metabolism[J]. J Biol Chem, 2001, 276(41): 37735-37738. DOI: 10.1074/jbc.R100035200. [13] GOODWIN B, JONES SA, PRICE RR, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis[J]. Mol Cell, 2000, 6(3): 517-526. DOI: 10.1016/s1097-2765(00)00051-4. [14] ZOLLNER G, MARSCHALL HU, WAGNER M, et al. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: Pathogenetic and therapeutic considerations[J]. Mol Pharm, 2006, 3(3): 231-251. DOI: 10.1021/mp060010s. [15] GIJBELS E, VINKEN M. Mechanisms of drug-induced cholestasis[J]. Methods Mol Biol, 2019, 1981: 1-14. DOI: 10.1007/978-1-4939-9420-5_1. [16] LEFEBVRE P, CARIOU B, LIEN F, et al. Role of bile acids and bile acid receptors in metabolic regulation[J]. Physiol Rev, 2009, 89(1): 147-191. DOI: 10.1152/physrev.00010.2008. [17] XU LJ, JIANG SD, JI H, et al. Role of bile acid nuclear receptor FXR in cholestasis and related pharmacotherapy[J]. J Pharm Res, 2018, 37(3): 169-173. DOI: 10.13506/j.cnki.jpr.2018.03.011.徐俐洁, 蒋思达, 季晖, 等. 胆汁酸核受体FXR在胆汁淤积及其药物治疗中的研究进展[J]. 药学研究, 2018, 37(3): 169-173. DOI: 10.13506/j.cnki.jpr.2018.03.011. [18] MARIN JJ, MACIAS RI, BRIZ O, et al. Bile acids in physiology, pathology and pharmacology[J]. Curr Drug Metab, 2015, 17(1): 4-29. DOI: 10.2174/1389200216666151103115454. 期刊类型引用(5)
1. 蔡纲,高庆娥,史元建. 富马酸替诺福韦酯和富马酸丙酚替诺福韦序贯联合聚乙二醇干扰素α-2b治疗慢性乙型肝炎患者的疗效比较. 黑龙江医药科学. 2024(01): 154-157 .  百度学术
百度学术2. 庄辉. 慢性乙型肝炎:从"只治"到"全治". 中华肝脏病杂志. 2024(05): 389-393 .  百度学术
百度学术3. 曾达武,朱月永. 非活动性HBsAg携带状态抗病毒治疗的利与弊. 临床内科杂志. 2023(12): 802-806 .  百度学术
百度学术4. 曾达武,朱月永. 非活动性乙型肝炎病毒表面抗原携带者的治疗. 国际流行病学传染病学杂志. 2022(04): 225-231 .  百度学术
百度学术5. 郑慧珂,孙昳,温瑾. 干扰素与核苷(酸)类似物对慢性乙型肝炎患者外周血调节性T细胞水平的影响. 中国实用医刊. 2021(04): 101-103 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 4120 KB)
PDF下载 ( 4120 KB)

 下载:
下载:






 百度学术
百度学术

