神经前体细胞表达发育下调蛋白4-1在胰腺癌组织中的表达和临床意义
DOI: 10.3969/j.issn.1001-5256.2022.03.024
伦理学声明:本研究方案于2020年2月20日经由徐州市中心医院伦理委员会审批通过,批号:2020伦审026号。
利益冲突声明:本研究不存在研究者、伦理委员会成员、受试者监护人以及与公开研究成果有关的利益冲突。
作者贡献声明:毕亭亭、刘岩负责设计研究思路,论文撰写; 毕亭亭、侯思慧负责收集临床资料,统计分析; 刘岩负责论文最终定稿。
Expression of neural precursor cell expressed developmentally downregulated 4-1 in pancreatic cancer tissue and its clinical significance
-
摘要:
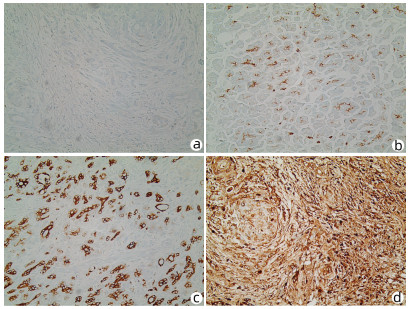
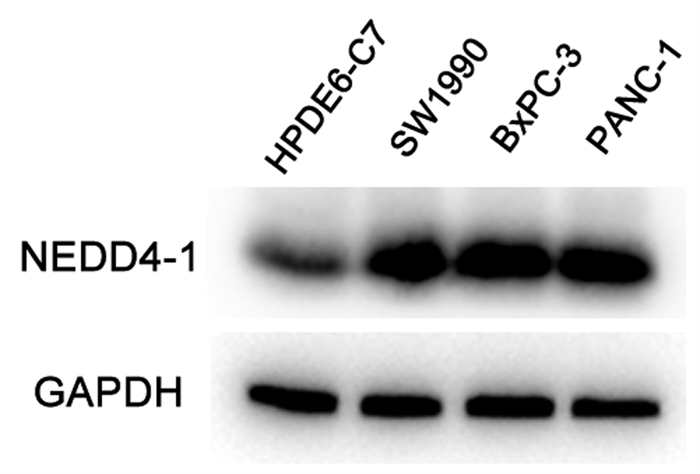
目的 探讨泛素化E3连接酶神经前体细胞表达发育下调蛋白4-1(NEDD4-1)在胰腺癌组织中的表达及临床意义。 方法 选取2017年1月—2019年12月于徐州市中心医院行手术治疗的病理学检查确诊为胰腺导管腺癌患者58例。采用免疫组化法检测胰腺癌组织标本中NEDD4-1的表达情况,分析NEDD4-1的表达与胰腺癌临床病理特征的相关性。使用Western Blot检测正常胰腺导管上皮HPDE6-C7细胞及胰腺癌SW1990、BxPC-3、PANC-1细胞中NEDD4-1的表达水平。计量资料两组间比较采用t检验。计数资料两组间比较采用χ2检验。采用Kaplan-Meier检验绘制生存曲线,log-rank检验进行生存分析。采用Cox回归分析预后相关因素。 结果 NEDD4-1在胰腺癌组织里的表达水平较癌旁组织中明显升高(79.31% vs 19.05%,χ2=35.614,P=0.001),且NEDD4-1在胰腺癌细胞株中的蛋白表达水平较正常胰腺导管上皮细胞明显上调(P值均<0.01)。NEDD4-1的表达分别与胰腺癌患者的远处转移、肿瘤分化程度和肿瘤TNM分期相关(χ2值分别为5.089、9.071、8.882,P值分别为0.04、0.003、0.003)。NEDD4-1阳性表达患者的平均存活时间明显短于阴性表达患者[(13.61±0.95)个月vs (22.22±2.20)个月,P=0.001]。Cox回归分析结果显示,NEDD4-1表达(HR=2.312,95% CI: 1.010~5.295,P=0.047)、淋巴结转移(HR=2.981,95% CI: 1.556~5.712,P=0.001)及肿瘤分化程度(HR=2.144,95% CI: 1.155~3.979,P=0.016)为胰腺癌患者预后的独立危险因素。 结论 NEDD4-1在胰腺癌组织及细胞内的阳性表达明显升高,且NEDD4-1阳性表达与预后不良相关,或可作为胰腺癌预后的一个预测因子和潜在治疗靶点。 Abstract:Objective To investigate the expression of the E3 ubiquitin ligase neural precursor cell expressed developmentally downregulated 4-1 (NEDD4-1) in pancreatic cancer tissue and its clinical significance. Methods Clinical data were collected from 58 patients who underwent surgical treatment in Xuzhou Central Hospital from January 2017 to December 2019 and were diagnosed with pancreatic ductal adenocarcinoma based on pathological examination. Immunohistochemistry was used to measure the expression of NEDD4-1 in pancreatic cancer tissue samples, and the association between the expression of NEDD4-1 and the clinicopathological features of pancreatic cancer was analyzed. Western blot was used to measure the protein expression level of NEDD4-1 in normal pancreatic ductal epithelial HPDE6-C7 cells and pancreatic cancer SW1990, BxPC-3, and PANC-1 cells. The t-test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between two groups. The Kaplan-Meier method was used to plot survival curves, and the log-rank test was used for survival analysis. The Cox proportional-hazards regression model was used to investigate the factors associated with prognosis. Results The expression level of NEDD4-1 in pancreatic cancer tissue was significantly higher than that in adjacent tissue (79.31% vs 19.05%, χ2=35.614, P < 0.01), and the protein expression of NEDD4-1 in pancreatic cancer cells was significantly higher than that in normal pancreatic ductal epithelial cells (P < 0.01). In the patients with pancreatic cancer, the expression of NEDD4-1 was associated with distant metastasis (χ2=5.089, P=0.040), tumor differentiation (χ2=9.071, P=0.003), and TNM stage (χ2=8.882, P=0.003). The patients with high NEDD4-1 expression had a significantly shorter mean survival time than those with low expression (13.61±0.95 months vs 22.22±2.20 months, P=0.001). The Cox regression analysis showed that NEDD4-1 expression (hazard ratio [HR]=2.312, 95% confidence interval [CI]: 1.010-5.295, P=0.047), degree of tumor differentiation (HR=2.981, 95% CI: 1.556-5.712, P=0.001), and lymph node metastasis (HR=2.144, 95% CI: 1.155-3.979, P=0.016) were independent risk factors for the prognosis of patients with pancreatic cancer. Conclusion There is a significant increase in the expression of NEDD4-1 in pancreatic cancer tissue and cells, and the high expression of NEDD4-1 is associated with poor prognosis. Therefore, it can be used as a prognostic biomarker and a therapeutic target for pancreatic cancer. -
Key words:
- Pancreatic Neoplasms /
- Ubiquitin-Protein Ligases /
- Pathology, Clinical /
- Prognosis
-
表 1 胰腺癌组织内NEDD4-1表达水平患者的临床病理特征比较
临床因素 例数 NEDD4-1表达 χ2值 P值 阳性 阴性 年龄[例(%)] 0.602 0.515 ≤60岁 20 17(85.0) 3(15.0) >60岁 38 29(76.3) 9(23.7) 性别[例(%)] 1.063 0.348 男 31 23(74.2) 8(25.8) 女 27 23(85.2) 4(14.8) 肿瘤大小[例(%)] 2.425 0.156 ≤4 cm 43 32(74.4) 11(25.6) >4 cm 15 14(93.3) 1(6.7) 淋巴结转移[例(%)] 2.825 0.115 有 31 22(71.0) 9(29.0) 无 27 24(88.9) 3(11.0) 远处转移[例(%)] 5.089 0.040 有 37 26(70.3) 11(29.7) 无 21 20(95.2) 1(4.8) 肿瘤分化程度[例(%)] 9.071 0.003 高/中分化 26 16(61.5) 10(38.5) 低分化 32 30(93.8) 2(6.2) TNM分期[例(%)] 8.882 0.003 Ⅰ+Ⅱ 31 20(64.5) 11(35.5) Ⅲ+Ⅳ 27 26(96.3) 1(3.7) 表 2 影响胰腺癌患者预后的Cox比例风险模型分析
参数 单因素分析 多因素分析 HR 95% CI P值 HR 95% CI P值 年龄 0.791 0.445~1.408 0.426 - - - 性别 1.107 0.634~1.933 0.721 - - - 肿瘤大小 1.324 0.602~2.916 0.485 - - - 远处转移 2.894 1.572~5.328 0.001 1.947 0.663~5.717 0.225 TNM分期 2.582 1.442~4.621 0.001 0.640 0.214~1.913 0.425 NEDD4-1表达 3.474 1.598~7.552 0.002 2.312 1.010~5.295 0.047 淋巴结转移 3.102 1.718~5.599 0.001 2.981 1.556~5.712 0.001 分化程度 1.912 0.946~3.041 0.036 2.144 1.155~3.979 0.016 -
[1] SIEGEL RL, MILLER KD, JEMAL A. Cancer statistics, 2018[J]. CA Cancer J Clin, 2018, 68(1): 7-30. DOI: 10.3322/caac.21442. [2] CHEN W, ZHENG R, BAADE PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2): 115-132. DOI: 10.3322/caac.21338. [3] SIEGEL RL, MILLER KD, JEMAL A. Cancer statistics, 2020[J]. CA Cancer J Clin, 2020, 70(1): 7-30. DOI: 10.3322/caac.21590. [4] SAAD AM, TURK T, AL-HUSSEINI MJ, et al. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study[J]. BMC Cancer, 2018, 18(1): 688. DOI: 10.1186/s12885-018-4610-4. [5] CHEN RF, ZHONG CR, ZHOU QB. Current status and perspectives of minimally invasive surgical treatment of pancreatic head carcinoma[J]. J Chin Hepatol, 2019, 35(5): 953-957. DOI: 10.3969/j.issn.1001-5256.2019.05.004.陈汝福, 钟诚锐, 周泉波. 胰头癌微创手术治疗的现状及展望[J]. 临床肝胆病杂志, 2019, 35(5): 953-957. DOI: 10.3969/j.issn.1001-5256.2019.05.004. [6] GE WY, WANG HX. Immunotherapy for pancreatic ductal adenocarcinoma: Challenges and opportunities[J]. J Clin Hepatol, 2019, 35(5): 958-963. DOI: 10.3969/j.issn.1001-5256.2019.05.005.葛伟玉, 王红霞. 胰腺导管腺癌的免疫治疗——挑战与机遇并存[J]. 临床肝胆病杂志, 2019, 35(5): 958-963. DOI: 10.3969/j.issn.1001-5256.2019.05.005. [7] CHEN Y, van de VIJVER MJ, HIBSHOOSH H, et al. PTEN and NEDD4 in human breast carcinoma[J]. Pathol Oncol Res, 2016, 22(1): 41-47. DOI: 10.1007/s12253-015-9971-2. [8] AMODIO N, SCRIMA M, PALAIA L, et al. Oncogenic role of the E3 ubiquitin ligase NEDD4-1, a PTEN negative regulator, in non-small-cell lung carcinomas[J]. Am J Pathol, 2010, 177(5): 2622-2634. DOI: 10.2353/ajpath.2010.091075. [9] SUN A, YU G, DOU X, et al. Nedd4-1 is an exceptional prognostic biomarker for gastric cardia adenocarcinoma and functionally associated with metastasis[J]. Mol Cancer, 2014, 13: 248. DOI: 10.1186/1476-4598-13-248. [10] YANG YM. Evaluation of American Joint Commission on Cancer (8th edition) and Japanese Pancreas Society(7th edition) changes for T and N staging in patients with pancreatic adenocarcinoma[J]. Chin J Surg, 2017, 55(1): 20-23. DOI: 10.3760/cma.j.issn.0529-5815.2017.01.006.杨尹默. AJCC第八版及日本胰腺学会第七版胰腺癌TNM分期的更新要点及内容评介[J]. 中华外科杂志, 2017, 55(1): 20-23. DOI: 10.3760/cma.j.issn.0529-5815.2017.01.006. [11] WANG D, CUI M, YU J. Research advances of precision treatment for pancreatic cancer[J]. Chin J Dig Surg, 2021, 20(4): 385-394. DOI: 10.3760/cma.J.cn115610-20210223-00089.汪栋, 崔铭, 余俊. 胰腺癌精准治疗的研究进展[J]. 中华消化外科杂志, 2021, 20(4): 385-394. DOI: 10.3760/cma.J.cn115610-20210223-00089. [12] WEI W, ZENG H, ZHENG R, et al. Cancer registration in China and its role in cancer prevention and control[J]. Lancet Oncol, 2020, 21(7): e342-342, e349. DOI: 10.1016/S1470-2045(20)30073-5. [13] FENG RM, ZONG YN, CAO SM, et al. Current cancer situation in China: Good or bad news from the 2018 Global Cancer Statistics?[J]. Cancer Commun (Lond), 2019, 39(1): 22. DOI: 10.1186/s40880-019-0368-6. [14] MAO N, HUANG ZJ, LIN ZT, et al. Current status of the application of translational medicine in the early diagnosis of pancreatic cancer[J]. Chin J Dig Surg, 2021, 20(4): 466-470. DOI: 10.3760/cma.j.cn115610-20210117-00029.毛宁, 黄子健, 林志涛, 等. 转化医学在胰腺癌早期诊断中的应用现状[J]. 中华消化外科杂志, 2021, 20(4): 466-470. DOI: 10.3760/cma.j.cn115610-20210117-00029. [15] MOGK A, SCHMIDT R, BUKAU B. The N-end rule pathway for regulated proteolysis: Prokaryotic and eukaryotic strategies[J]. Trends Cell Biol, 2007, 17(4): 165-172. DOI: 10.1016/j.tcb.2007.02.001. [16] BALZAC F, AVOLIO M, DEGANI S, et al. E-cadherin endocytosis regulates the activity of Rap1: A traffic light GTPase at the crossroads between cadherin and integrin function[J]. J Cell Sci, 2005, 118(Pt 20): 4765-4783. DOI: 10.1242/jcs.02584. [17] YANG CL, LI QQ, XIA RL, et al. Effects of NEDD4 on proliferation, migration, and invasion of bladder cancer cells[J]. Shandong Med J, 2019, 59(16): 37-40. DOI: 10.3969/j.issn.1002-266X.2019.16.010.杨传来, 李巧巧, 夏任兰, 等. NEDD4基因沉默对膀胱癌细胞增殖、迁移和侵袭的影响[J]. 山东医药, 2019, 59(16): 37-40. DOI: 10.3969/j.issn.1002-266X.2019.16.010. [18] LUHTALA S, STAFF S, KALLIONIEMI A, et al. Clinicopathological and prognostic correlations of HER3 expression and its degradation regulators, NEDD4-1 and NRDP1, in primary breast cancer[J]. BMC Cancer, 2018, 18(1): 1045. DOI: 10.1186/s12885-018-4917-1. [19] WENG M, LUO ZL, WU XL, et al. The E3 ubiquitin ligase NEDD4 is translationally upregulated and facilitates pancreatic cancer[J]. Oncotarget, 2017, 8(12): 20288-20296. DOI: 10.18632/oncotarget.15446. 期刊类型引用(10)
1. 雷后胜. 奥曲肽联合加大补液量在肝硬化难治性腹水中的作用. 航空航天医学杂志. 2024(12): 1450-1453 .  百度学术
百度学术2. 王慧玲,丁晓. 早期限制性液体复苏对肝硬化上消化道出血的复苏成功率及预后影响. 青岛医药卫生. 2022(01): 50-52 .  百度学术
百度学术3. 卢春燕,吴雄健. 肝硬化腹水的药物疗法. 赣南医学院学报. 2022(04): 396-398 .  百度学术
百度学术4. 杨梅,王谦. 奥曲肽联合西咪替丁治疗上消化道出血患者的效果及对止血时间、血小板计数的影响. 临床医学研究与实践. 2021(07): 66-68 .  百度学术
百度学术5. 刘聪. 奥曲肽联合奥美拉唑对上消化道出血的疗效探讨. 系统医学. 2021(02): 56-58 .  百度学术
百度学术6. 刁利霞,莫冬林,吴宇平. 奥曲肽结合加大补液量治疗肝硬化难治性腹水的效果及对其血清学指标的影响. 北方药学. 2020(01): 55-56 .  百度学术
百度学术7. 张兵,邓丹. 肝硬化难治性腹水的临床治疗效果分析. 中国继续医学教育. 2019(05): 113-115 .  百度学术
百度学术8. 马陈斌. 肝硬化腹水的临床治疗应用效果观察. 世界最新医学信息文摘. 2019(27): 72+83 .  百度学术
百度学术9. 何清辉. 前列地尔辅助治疗肝硬化难治性腹水的可行性分析. 慢性病学杂志. 2019(07): 1039-1041 .  百度学术
百度学术10. 李青,孙咏红. 托伐普坦治疗顽固性腹水患者临床疗效及安全性. 中国实用医药. 2018(32): 131-133 .  百度学术
百度学术其他类型引用(1)
-




 PDF下载 ( 3129 KB)
PDF下载 ( 3129 KB)

 下载:
下载:

 百度学术
百度学术

